A Preliminary Study of the Resting-State Speech Rate Network in Patients With Parkinson’s Disease


Copyright 2020 ⓒ Korean Speech-Language & Hearing Association.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Studies on the rate of speech in patients with Parkinson’s disease (PD) report various results. Some results show slower speech rate in patients with PD than in normal controls (NC), while others report an increased rate of speech in these; others still, reveal no differences between the NC and PD groups. Despite our understanding of the sites primarily responsible for speech production in NC, alteration of the functional connectivity (FC) in patients with PD still remains unclear. The aim of this study was to map the speech rate-dependent neural connections using resting-state (rs) FC techniques.
This study included 14 patients with PD and 10 NC. After testing diadochokinetic rate, all participants underwent rs functional magnetic resonance imaging (fMRI) scanning. We then selected four seeds in each subcortical region based on prior fMRI studies, especially the basal ganglia and cerebellum.
Compared with the NC group, all the seed regions of interest (ROIs) in the PD group, except the left putamen and right caudate nucleus, showed significantly decreased or increased FC with the cortical and subcortical areas. Among these ROIs, only the left cerebellum (lobule VI) exhibited significantly increased FC with the right inferior parietal lobule in the PD group. While functional connectivity is altered, the speech performance in patients with PD remained similar to that of the NC group.
Using the rs fMRI technique, we provided evidence that the left cerebellum is strongly interconnected with the right cerebral cortex in patients with PD. We propose that activation of the cerebellum participates in the compensatory mechanism which underlies the lack of statistically significant difference in speech rate when patients with PD are compared with NC, although overall FC decreases.
초록
파킨슨병(Parkinson’s disease: PD)으로 인해 발현되는 운동저하형 마비말장애(hypokinetic dysarthria)는 구어를 사용한 의사소통 능력을 감소시켜 환자의 삶의 질을 저하시키는데, PD에서 기인된 마비말장애는 타 마비말장애에서는 관찰하기 힘든 이질적인 말속도 유형(느려진, 빨라진 혹은 변화 없는 말속도)이 집단 내에서 존재한다. 전통적으로 기저핵 이상이 PD 관련 운동 문제의 원인으로 알려져 왔지만 근래에 이루어진 뇌영상 연구는 소뇌 관련성을 점차적으로 강조하고 있다. 이에 파킨슨병 환자의 말속도 변화를 기저핵뿐만 아니라 소뇌를 포함시켜 살펴볼 필요성이 제기된다.
약물이 효과적으로 작용하는 상태의 파킨슨병 환자 14명(남자 : 여자=8 : 6)과 정상 통제군 10명(남자 : 여자=4 : 6)의 뇌영상을 3.0 테슬라를 사용하여 휴식 상태에서 획득한 뒤 말속도 조절에 관여하는 주요 피질하 영역(기저핵; 꼬리핵의 머리, 조가비핵, 소뇌; VI, VIIIA)으로 알려진 곳을 관심영역(region of interests: ROIs)으로 지정하여 전체 뇌에서 군 간의 기능 연결성을 확인하였다. 그리고 교호운동속도(diadochokinetic rates: DDK rates) 과제인 교대운동속도(alternating motion rates: AMRs)와 일련운동속도(sequential motion rates: SMRs)를 실시하여 두 집단 간의 말속도를 비교하였다.
첫째, 집단 간 비교에서 환자군은 왼쪽 소뇌 VI-오른쪽 하두정엽을 제외한 뇌 전반에서 통제군에 비해 기능적 활성화가 유의미하게 저하되었다. 둘째, DDK 과제에서 두 집단 간의 말속도는 통계적으로 유의미한 차이가 없었다.
전반적으로 저하된 뇌 기능에도 불구하고 파킨슨병 환자군이 보여준 정상군과 유사한 수준의 말속도 수행은 발병 후 변화 없는 말속도를 보이는 환자군의 경우는 기능적 연결성이 증가한 왼쪽 소뇌와 오른쪽 하두정엽의 작용에 따른 결과일 가능성이 제기되었다.
Keywords:
Parkinson’s disease, speech rate, resting-state functional magnetic resonance imaging, cerebellum, basal ganglia키워드:
파킨슨병, 말속도, 휴식상태 뇌기능자기공명영상, 소뇌, 기저핵Ⅰ. Introduction
Speech is one of the most intricate and motoric human behaviors with respect to the involved neural networks. Although brain areas related to speech production have been well-investigated in normal subjects (Eickhoff et al., 2009; Guenther et al., 2006; Price, 2012; Simonyan & Fuertinger, 2015), our understanding of altered neural networks originated from the basal ganglia pathology in patients with PD still remains unclear. Traditionally, sequences of the events involved in speech production, such as planning, programming, and execution, were thought to be controlled mainly by the cerebral cortex, with the subcortical areas playing a minor role in the selection of desired movements and / or the inhibition of unwanted movements (Albin et al., 1989; Mink, 1996).
However, over the past 20 years, such a distinct separation of roles of the brain structures in controlling speech production has been challenged by new information. Using various brain imaging technologies, the interconnection between the cortical and subcortical areas to control of speech motor, especially the basal ganglia and cerebellum, have been highlighted (Bohland & Guenther, 2006; Riecker et al., 2005; Wildgruber et al., 2001). Neuroimaging studies on normal speech motor control have delineated a “minimal network for overt speech production” including the mesiofrontal structures (supplementary motor area and anterior cingulate gyrus), bilateral pre- and post- central convolutions, extending rostrally into posterior parts of the inferior frontal gyrus of the language-dominant hemisphere and left anterior insula as well as bilateral components of the basal ganglia, cerebellum, and thalamus (Bohland & Guenther, 2006).
However, despite our understanding of the sites primarily responsible for speech production in normal subjects, alteration of the functional network in patients with PD still remains unclear. Although often veiled by the more prominent and distressing skeletal aspects such as gait and upper limb control, speech impairment is a very frequent symptom in PD. It is estimated that approximately 70% (Hartelius & Svensson, 1994), with some estimates reaching as high as 90% (New et al., 2015; Ramig et al., 2011), patients with PD undergo a disruption of speech motor control known as “hypokinetic dysarthria”. During disease progression, hypokinetic dysarthria can emerge at all stages of the disease, and generally worsens as the disease progresses, causing a progressive loss of communication abilities and increasing isolation from society (Pinto et al., 2004; Skodda, 2011).
Results of similar studies have demonstrated that patients with PD show abnormalities in voice production, so-called “hypophonia”; however, data concerning speech rate in these patients is insufficient and inconsistent. Studies on the rate of speech report various results, with some reporting slower speech rate in patients with PD than in normal speakers (Dworkin & Aronson, 1986; Hammen & Yorkston, 1996; Ludlow et al., 1987), while others report an increased rate of speech in these patients (Ackermann et al., 1995; Hirose et al., 1982; McRae et al., 2002), and yet others reveal no differences between the control and PD groups (Canter, 1965; Lowit et al., 2006; Skodda et al., 2011).
Traditionally, the classical model of PD (cortico-basal ganglia-thalamo-cortical loops) accentuated the function of the basal ganglia in the cardinal symptoms and axial features associated with PD. However, other changes in motor symptoms, such as speech, cannot be interpreted solely based on basal ganglia dysfunction. Limitations in the current hypothesis led us to believe that a large amount of evidence from experimental studies could be analyzed to delineate and understand ambiguous speech symptoms.
Previous resting-state (rs) studies on speech impairment in PD have mainly focused on the basal ganglia; therefore, we sought to investigate whether FC within the cerebellum might also be linked to speech rate impairment. Basal ganglia pathology may show that changes in the FC between basal ganglia structures and the cerebellum may also be involved in speech rate impairment in PD. While several studies have identified abnormal rs connections in the basal ganglia or at the cortical levels in PD, it remains unclear whether speech rate impairment in PD involves connectivity changes in the cerebellum. Furthermore, only a few studies have been conducted to assess neural connections based on connectivity in speech rate networks.
Our goal in this study was to map the speech rate-dependent neural connections using rs FC techniques. Rs networks are associated with self-oriented mental activity and offer a means of evaluating the status of functional systems within the brain, without external goal-directed cognitive performance. Therefore, the use of rs networks analysis may be advantageous for the identification of brain regions functionally involved in the pathological changes in neurodegenerative diseases (Filippi et al., 2018; Fox & Greicius, 2010). Thus, we performed a comparative analysis of rs FC in patients with PD and normal controls (NC) to further elucidate cerebellum-related alteration of FC.
Ⅱ. Methods
1. Participants
This study included 14 patients diagnosed with PD (men : women=8 : 6) and 10 healthy NC (men : women=4 : 6) who were recruited from a university hospital in Seoul between February and December of 2016. Diagnosis of PD was performed according to the clinical diagnostic criteria of the UK PD Society Brain Bank, and all participants’ native language was Korean.
Before the functional image acquisition, all participants were screened using cognition, depression, and language tests. The purpose of this study was to compare rs fMRI networks based on speech rate; therefore, factors that might negatively affect or influence speech rate needed to be controlled for before acquiring brain images. All participants who underwent fMRI scanning were found to be within the normal range (above the cut-off point based on years of education and age) on the Korean version of the Montreal Cognitive Assessment (K-MoCA, Kang et al., 2009), with a score < 16 on the Korean version of the Geriatric Depression Rating Scale (GDS-K, Cho et al., 1999), within 1 standard deviation (SD) of the Korean version of the Boston Naming Test (K-BNT, Kim & Na, 1997), and within 1 SD of Paradise Korean version of Western Aphasia Battery (PK-WAB, Kim & Na, 2012). To compare demographic characteristics of the two groups, the Mann-Whitney U-test and chi-squared (χ2) tests were used for continuous and categorical variables, respectively. Table 1 summarizes the demographic features of patients with PD and NC. No significant differences were found in age, sex, aphasia quotient, K-BNT, K-MoCA, and GDS-K scores between the PD and NC groups, except for years of education.
We received approval form the Yonsei University Severance Hospital ethical standards committee on human experimentation for experiments involving human subjects (IRB No. 4-2015-0843). Written informed consent was obtained from all the subjects who participated in this study.
2. Procedures
Speech data was acquired using diadochokinetic (DDK) rates. The DDK rates are composed of two types of rates: alternating motion rates (AMRs) and sequential motion rates (SMRs). Syllable repetitions, performed as fast as possible, have been demonstrated to be a valid test of maximum speaking rates, and have a potential relationship with speaking rates in more complex speech tasks, such as reading or conversation (Wang et al., 2004). Participants who produced rapid DDK rates at the expense of precision were instructed to speak as fast as possible without being imprecise. Each subject performed each task three times in a random order.
The speech recording procedure was as follows. All patients in the PD group were in “ON” medication state (Madhyastha et al., 2015; Martínez-Sánchez et al., 2016; New et al., 2015; Skodda, 2011). Because the aim of this study was to identify the brain functional network involved in speech production, and previous fMRI and perceptual evaluation studies have shown speech production to be similar with and without medication (Maillet et al., 2012; Skodda et al., 2011; Skodda et al., 2011; Skodda et al., 2010). Generally, patients with PD receive their medicine 3~4 times a day when they are awake; therefore, speech communication occurs only during the “ON” state. The speech samples were obtained using an electret condenser microphone (ECM-MS 908C; Sony, Japan) placed on a microphone stand approximately 10~15 cm from each participant’s mouth at 15°. The microphone was connected to a linear PCM-M10 recorder (Sony, Japan), and the acoustic signal was recorded at a sampling rate of 44.1 kHz, with 16-bit quantization.
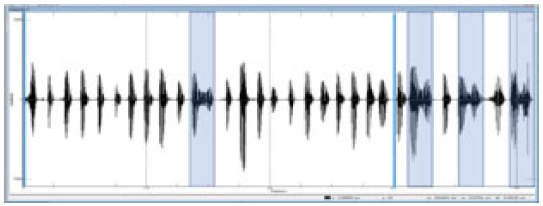
We captured 3 stable seconds from the overall duration (5 seconds) of the speech task using the Computerized Speech Lab (model 4150B; KayPENTAX, Lincoln Park, USA). If slurred speech was included in the 3 seconds, a spectrogram was used to determine a syllable; if it was not made up of a consonant-vowel (e.g., a consonant without a vowel or a vowel without a consonant), it was not counted as one syllable (Figure 1).
All participants underwent fMRI scanning with a 3.0-Tesla MRI scanner (Achieva, Philips Medical System, Best, Netherlands) to obtain T2*-weighted single-shot echo planar imaging sequences. Each participant was axially scanned using the following parameters: voxel size, 2.8 × 2.8 × 3.0 mm3; slice number, 31 (interleaved); matrix, 80 × 80; slice thickness, 3.0 mm; gap, 1.0 mm; repetition time, 2000 ms; echo time, 30 ms; flip angle=90°; and field of view, 220 mm. Each 330-second scan produced 165 fMR images. Earplugs were offered to the subjects to reduce the scanner noise. The subjects were instructed to remain awake and still, keep their eyes closed, and not focus on a specific thought. During the routine questioning performed immediately after the examination, none of the participants reported having fallen asleep (van den Heuvel & Hulshoff Pol, 2010).
Preprocessing of the fMRI data was conducted using the Analysis of Functional NeuroImages (https://afni.nimh.nih.gov/afni) software. To stabilize the magnetic field, the initial five volumes of each rs fMRI datum were excluded. The remaining fMRI data were processed in the following order: despiking, slice-timing, and head motion correction. Prior to the normalization of fMRI data to the common space, co-registration between anatomical T1 image and fMRI data was performed using the affine transform with Local Pearson Correlation cost function (Saad et al., 2009). Further, eroded white matter (WM) and large ventricle (LV) masks were registered to fMRI data using the same transform matrix. The fMRI data, anatomical T1 image, and masks in the native space were normalized to the common space using the standard Montreal Neurological Institute (MNI) 152 template with an isotropic voxel size of 2 mm. In addition, fMRI data were spatially blurred using a 6-mm full width at half maximum Gaussian kernel.
After spatial normalization, the fMRI data were corrected by nuisance-removal regression using the anatomy-based correlation corrections method (Jo et al., 2010). which is a regression model for extracting the nuisance signal of specific tissues, such as the WM and LV. It is assumed that the actual brain signal is generated from the gray matter. In order to avoid mixing signals from different tissues, anatomy-based regressors were created before spatial smoothing. The regressors of the anatomy-based correlation corrections method were as follows: (1) six parameters from the head motion correction, (2) locally (r=15 mm) averaged signal from the eroded WM mask, and (3) averaged signal from the eroded LV mask. The nuisance regression model was applied using censoring and bandpass filtering together. The censoring was applied to fMRI volumes with the Euclidean norm of the first derivative of head motion greater than .25. The bandpass filtering was applied to fMRI data with .009 < f < .08 to deduct physiological noise.
As we were interested in the key brain regions controlling speech rate, we selected four seed regions of interest (ROIs) in each hemisphere which were based on previous studies on speech production (Brown et al., 2009; Chang et al., 2009; New et al., 2015): (1) the putamen, (2) head of the caudate nucleus (3) lobule VI, and (4) lobule VIIIA of the cerebellum (Table 2). The averaged signal of each ROI was used as the reference signal. Each ROI correlation map was calculated using Pearson’s correlation coefficient with a reference signal for the whole brain. The correlation map for each subject was converted to a z-score map using Fisher’s r-to-z transformation.
3. Statistical Analysis
To compare the number of syllables per second (SPS) of DDK between the two groups, a Mann-Whitney U-test was conducted. Statistical analyses were performed using SPSS 23.0 software, and the significance level was set at .05 for all statistical analyses.
For comparison of FC between the two groups, a two-sample t-test was used while controlling for the effects of age and sex as covariates. To obtain the significance level combination of uncorrected individual voxel p-value and cluster size, Monte Carlo simulation was conducted to control for Type I errors, using the AlphaSim program (parameters: uncorrected individual voxel p-value=.02, simulation=10,000 times, 8-mm full width at half maximum Gaussian filter with a whole-brain mask (Poline et al., 1997). The two-sample t-test was corrected by p<.05 level (uncorrected individual voxel height threshold of p<.02, with a minimum cluster size of 307 voxels). Furthermore, to determine whether the observed FC could be used to discriminate between each group, Spearman’s partial correlations were used after controlling for age, sex, and years of education.
Ⅲ. Results
1. Functional Connectivity
Direct mean comparisons demonstrated that the patients with PD in “ON” medication state had decreased whole-brain FC (Table 3), i.e., between the basal ganglia and cerebral cortex (right putamen-left inferior parietal lobule and left head of caudate-left lingual gyrus), cerebellum and cerebral cortex (left cerebellum [lobule VI]-right cuneus, right cerebellum [lobule VI]-left superior temporal gyrus, right cerebellum [lobule VI]-right precentral gyrus, right cerebellum [lobule VI]-right postcentral gyrus, right cerebellum [lobule VI]-right precuneus, left cerebellum [lobule VIIIA]-right precentral gyrus, right cerebellum [lobule VIIIA]-right medial frontal gyrus, right cerebellum [lobule VIIIA]-left posterior cingulate gyrus), basal ganglia and cerebellum (left head of the caudate nucleus-right declive), and within the cerebellum (left cerebellum [lobule VI]-right declive, right cerebellum [lobule VI]-left culmen, and right cerebellum [lobule VIIIA]-left cerebellar tonsil), except the left cerebellum [lobule VI]-right inferior parietal lobule connection, compared with the controls.
2. DDK Rate
The median of SPS in the PD group measured was: 6.33 (interquartile range [IQR]: 1.37), 6.33 (IQR: 1.75), and 6.16 (IQR: 1.33) for /puh/, /tuh/, and /kuh/, respectively, while that for the NC group was: 6.83 (IQR: 1.17), 6.50 (IQR: .75), and 6.33 (IQR: .75) for /puh/, /tuh/, and /kuh/, respectively (Table 4). There was no significant difference between the two groups (p >.05). When comparing the median SMRs, the performance for /puhtuhkuh/ between the PD (median: 2.36, IQR: .67) and NC (median: 2.25, IQR: .41) groups was not statistically significantly different (p >.05, Table 5).
Ⅳ. Discussion
Speech is one of the most intricate and motoric human behaviors with respect to the involved neural networks. Although brain areas related to speech production have been well-investigated in normal subjects (Eickhoff et al., 2009;Guenther et al., 2006; Price, 2012; Simonyan & Fuertinger, 2015), our understanding of altered neural networks originated from the basal ganglia pathology in patients with PD still remains unclear. We used seed-based interregional correlations of rs fMRI data and investigated the functional interaction of the key brain regions related to speech rate, i.e., the putamen, head of the caudate nucleus, and cerebellum (lobules VI and VIIIA).
Compared with the NC group, all the seed ROIs in the PD group, except the left putamen and right caudate nucleus, showed significantly decreased or increased FC with the cortical and subcortical areas. Among these ROIs, only the left cerebellum (lobule VI) exhibited significantly increased FC with the right inferior parietal lobule in the PD group. This finding is consistent with previous studies showing that the brain function of patients with PD under dopaminergic medication is globally reduced compare with normal subjects (Berding et al., 2001; Festini et al., 2015; Maillet et al., 2012; New et al., 2015). This result highlights the fact that medication does not restore the FC in patients with PD to the normal state (Maillet et al., 2012). Nevertheless, this study results demonstrate that there is no difference in the median scores of speech parameters (i.e., AMRs, SMRs) when patients with PD are compared with NC. Although there were no relevant differences between the two groups, all the speech parameters presented a higher IQR in patients with PD.
One interpretation of this result is a cerebellar compensatory phenomenon to preserve speech rate in PD. The cerebellum and basal ganglia are the two major subcortical structures that influence multiple aspects of motor behavior (Strick et al., 2009). Both structures form multi-synaptic loops; moreover, substantial interactions exist between these structures, which are linked together to produce an integrated function (Bostan & Strick, 2010; Houk & Wise, 1995; Wu & Hallett, 2013). In particular, the cerebellum is thought to act a core role in the formation of internal representations for actions that allow us to execute precise temporal coordination, online sequencing, and smooth motor responses (Ackermann, 2008; Ackermann et al., 2007; Ito, 2008; Manto et al., 2012; Moberget & Ivry, 2016; Ramnani, 2006). Within the speech production system, one of the main functions of the cerebellum is to control the regulation of the ongoing temporal sequencing, not only during overt speaking but also the generation of the prearticulatory verbal cord in covert or silent speech production (Callan et al., 2006; Gordon, 1996; Simonyan & Fuertinger, 2015).
Additionally, as previously mentioned, compared with the NC group, the PD group had significantly increased strength of FC between the left cerebellar lobule VI and right inferior parietal lobule. A recent study suggested that the function connection between the cerebellum and inferior parietal lobule plays critical roles in the formation of the speech production network (Simonyan & Fuertinger, 2015). The inferior parietal lobule is known to be involved in phonological processing (Hickok & Poeppel, 2007), monitoring the onset of speech (Kort et al., 2014), and guiding of movement via sensory feedback (Mattingley et al., 1998), which may be critical to facilitate the transition from the resting state to speaking (Simonyan & Fuertinger, 2015) and to execute speech production (Hallett & Grafman, 1997).
Another interpretation considers the inferior parietal lobule to be a primary region for sensorimotor integration, functioning as an “association cortex”. Patients of PD exhibit sensorimotor integration impairments from the early stage of the disease. Thus, the hyper-activity of the inferior parietal lobule might be a strategy to compensate for impaired processing of the somatosensory input via the pathologically affected basal ganglia toward the motor cortex (Tahmasian et al., 2017).
In using the Rs fMRI technique, we provided evidence that the PD subjects exhibited a significant decrease in functional connectivity than the NC group and proved the specific cerebellum area was associated with speech rate control. The results would provide a better understanding of the disease and provide information for the development of new treatment approaches.
Acknowledgments
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (No. NRF-2015S1A5A8017149).
이 논문은 2015년 대한민국 교육부와 한국연구재단의 지원을 받아 수행된 연구임(No. NRF-2015S1A5A8017149).
References
-
Ackermann, H. (2008). Cerebellar contributions to speech production and speech perception: Psycholinguistic and neurobiological perspectives. Trends in Neurosciences, 31(6), 265-272.
[https://doi.org/10.1016/j.tins.2008.02.011]

-
Ackermann, H., Hertrich, I., & Hehr, T. (1995). Oral diadochokinesis in neurological dysarthrias. Folia Phoniatrica Logopaedica, 47(1), 15-23.
[https://doi.org/10.1159/000266338]

-
Ackermann, H., Mathiak, K., & Riecker, A. (2007). The contribution of the cerebellum to speech production and speech perception: Clinical and functional imaging data. The Cerebellum, 6(3), 202-213.
[https://doi.org/10.1080/14734220701266742]

-
Albin, R. L., Young, A. B., & Penney, J. B. (1989). The functional anatomy of basal ganglia disorders. Trends in Neurosciences, 12(10), 366-375.
[https://doi.org/10.1016/0166-2236(89)90074-X]

-
Berding, G., Odin, P., Brooks, D. J., Nikkhah, G., Matthies, C., Peschel, T., . . . Knapp, W. H. (2001). Resting regional cerebral glucose metabolism in advanced Parkinson's disease studied in the off and on conditions with [(18)F]FDG-PET. Movement Disorders: Official Journal of the Movement Disorder Society, 16(6), 1014-1022.
[https://doi.org/10.1002/mds.1212]

-
Bohland, J. W., & Guenther, F. H. (2006). An fMRI investigation of syllable sequence production. Neuroimage, 32(2), 821-841.
[https://doi.org/10.1016/j.neuroimage.2006.04.173]

-
Bostan, A. C., & Strick, P. L. (2010). The cerebellum and basal ganglia are interconnected. Neuropsychology Review, 20(3), 261-270.
[https://doi.org/10.1007/s11065-010-9143-9]

-
Brown, S., Laird, A. R., Pfordresher, P. Q., Thelen, S. M., Turkeltaub, P., & Liotti, M. (2009). The somatotopy of speech: Phonation and articulation in the human motor cortex. Brain and Cognition, 70(1), 31-41.
[https://doi.org/10.1016/j.bandc.2008.12.006]

-
Callan, D. E., Tsytsarev, V., Hanakawa, T., Callan, A. M., Katsuhara, M., Fukuyama, H., & Turner, R. (2006). Song and speech: Brain regions involved with perception and covert production. Neuroimage, 31(3), 1327-1342.
[https://doi.org/10.1016/j.neuroimage.2006.01.036]

-
Canter, G. J. (1965). Speech characteristics of patients with Parkinson’s disease: III. Articulation, diadochokinesis, and over-all speech adequacy. Journal of Speech and Hearing Disorders, 30(3), 217-224.
[https://doi.org/10.1044/jshd.3003.217]

-
Chang, S. E., Kenney, M. K., Loucks, T. M., Poletto, C. J., & Ludlow, C. L. (2009). Common neural substrates support speech and non-speech vocal tract gestures. Neuroimage, 47(1), 314-325.
[https://doi.org/10.1016/j.neuroimage.2009.03.032]

- Cho, M. J., Bae, J. N., Suh, G. H., Hahm, B. J., Kim, J. K., Lee, D. W., & Kang, M. H. (1999). Validation of geriatric depression scale, Korean version (GDS) in the assessment of DSM-Ⅲ-R major depression. Journal of Korean Neuropsychiatric Association, 38(1), 48-63.
-
Dworkin, J. P., & Aronson, A. E. (1986). Tongue strength and alternate motion rates in normal and dysarthric subjects. Journal of Communication Disorders, 19(2), 115-132.
[https://doi.org/10.1016/0021-9924(86)90015-8]

-
Eickhoff, S. B., Heim, S., Zilles, K., & Amunts, K. (2009). A systems perspective on the effective connectivity of overt speech production. Physical and Engineering Sciences, 367(1896), 2399-2421.
[https://doi.org/10.1098/rsta.2008.0287]

-
Festini, S. B., Bernard, J. A., Kwak, Y., Peltier, S., Bohnen, N. I., Muller, M. L., Dayalu, P., & Seidler, R. D. (2015). Altered cerebellar connectivity in Parkinson's patients ON and OFF L-DOPA medication. Frontiers in Human Neuroscience, 9, 214.
[https://doi.org/10.3389/fnhum.2015.00214]

-
Filippi, M., Elisabetta, S., Piramide, N., & Agosta, F. (2018). Functional MRI in idiopathic Parkinson's disease. International Review of Neurobiology, 141, 439-467.
[https://doi.org/10.1016/bs.irn.2018.08.005]

-
Fox, M. D., & Greicius, M. (2010). Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience, 4, 19.
[https://doi.org/10.3389/fnsys.2010.00019]

-
Gordon, N. (1996). Speech, language, and the cerebellum. International Journal of Language & Communication Disorders, 31(4), 359-367.
[https://doi.org/10.3109/13682829609031327]

-
Guenther, F. H., Ghosh, S. S., & Tourville, J. A. (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96(3), 280-301.
[https://doi.org/10.1016/j.bandl.2005.06.001]

-
Hallett, M., & Grafman, J. (1997). Executive function and motor skill learning. International Review of Neurobiology, 41, 297-323.
[https://doi.org/10.1016/S0074-7742(08)60357-8]

-
Hammen, V. L., & Yorkston, K. M. (1996). Speech and pause characteristics following speech rate reduction in hypokinetic dysarthria. Journal of Communication Disorders, 29(6), 429-444.
[https://doi.org/10.1016/0021-9924(95)00037-2]

-
Hartelius, L., & Svensson, P. (1994). Speech and swallowing symptoms associated with Parkinson's disease and multiple sclerosis: A survey. Folia Phoniatrica et Logopaedica, 46, 9-17.
[https://doi.org/10.1159/000266286]

-
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393-402.
[https://doi.org/10.1038/nrn2113]

-
Hirose, H., Kiritani, S., & Sawashima, M. (1982). Velocity of articulatory movements in normal and dysarthric subjects. Folia Phoniatrica, 34(4), 210-215.
[https://doi.org/10.1159/000265651]

-
Houk, J. C., & Wise, S. P. (1995). Feature article: Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning ad controlling action. Cerebral Cortex, 5(2), 95-110.
[https://doi.org/10.1093/cercor/5.2.95]

-
Ito, M. (2008). Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci, 9(4), 304-313.
[https://doi.org/10.1038/nrn2332]

-
Jo, H. J., Saad, Z. S., Simmons, W. K., Milbury, L. A., & Cox, R. W. (2010). Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage, 52(2), 571-582.
[https://doi.org/10.1016/j.neuroimage.2010.04.246]

-
Kang, Y. W., Park, J. S., Yu, K. H., & Lee, B. C. (2009). A reliability, validity, and normative study of the Korean-Montreal Cognitive Assessment (K-MoCA) as an instrument for screening of vascular cognitive impairment (VCI). Korean Journal of Clinical Psychology, 28(2) 549-562.
[https://doi.org/10.15842/kjcp.2009.28.2.013]

- Kim, H. H., & Na, D. L. (1997). Korean version-Boston Naming Test. Seoul: Hakjisa.
- Kim, H. H., & Na, D. L. (2012). ParadiseㆍKorean version-Western Aphasia Battery-revised. Seoul: Paradise.
-
Kort, N. S., Nagarajan, S. S., & Houde, J. F. (2014). A bilateral cortical network responds to pitch perturbations in speech feedback. Neuroimage, 86, 525-535.
[https://doi.org/10.1016/j.neuroimage.2013.09.042]

- Lowit, A., Brendel, B., Dobinson, C., & Howell, P. (2006). An investigation into the influences of age, pathology and cognition on speech production. Journal of Medical Speech-Language Pathology, 14, 253-262. PMCID: PMC2661059
-
Ludlow, C. L., Connor, N. P., & Bassich, C. J. (1987). Speech timing in Parkinson's and Huntington's disease. Brain and Language, 32(2), 195-214.
[https://doi.org/10.1016/0093-934X(87)90124-6]

-
Madhyastha, T. M., Askren, M. K., Zhang, J., Leverenz, J. B., Montine, T. J., & Grabowski, T. J. (2015). Group comparison of spatiotemporal dynamics of intrinsic networks in Parkinson's disease. Brain, 138(9), 2672-2686.
[https://doi.org/10.1093/brain/awv189]

-
Maillet, A., Krainik, A., Debû, B., Troprès, I., Lagrange, C., Thobois, S., . . . Pinto, S. (2012). Levodopa effects on hand and speech movements in patients with Parkinson’s disease: A FMRI study. PLoS One, 7(10).
[https://doi.org/10.1371/journal.pone.0046541]

-
Manto, M., Bower, J. M., Conforto, A. B., Delgado-García, J. M., Da Guarda, S. N. F., Gerwig, M., . . . Molinari, M. (2012). Consensus paper: Roles of the cerebellum in motor control-the diversity of ideas on cerebellar involvement in movement. The Cerebellum, 11(2), 457-487.
[https://doi.org/10.1007/s12311-011-0331-9]

-
Martínez-Sánchez, F., Meilán, J. J. G., Carro, J., Gómez Íñiguez, C., Millian-Morell, L., Pujante Valverde, I. M., . . . López, D. E. (2016). Speech rate in Parkinson's disease: A controlled study. Neurología (English edition), 31(7), 466-472.
[https://doi.org/10.1016/j.nrleng.2014.12.014]

-
Mattingley, J. B., Husain, M., Rorden, C., Kennard, C., & Driver, J. (1998). Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature, 392(6672), 179-182.
[https://doi.org/10.1038/32413]

-
McRae, P. A., Tjaden, K., & Schoonings, B. (2002). Acoustic and perceptual consequences of articulatory rate change in Parkinson disease. Journal of Speech, Language, and Hearing Research, 45(1), 35-50.
[https://doi.org/10.1044/1092-4388(2002/003)]

-
Mink, J. W. (1996). The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology, 50(4), 381-425.
[https://doi.org/10.1016/S0301-0082(96)00042-1]

-
Moberget, T., & Ivry, R. B. (2016). Cerebellar contributions to motor control and language comprehension: Searching for common computational principles. Annals of the New York Academy of Sciences, 1369(1), 154-171.
[https://doi.org/10.1111/nyas.13094]

-
New, A. B., Robin, D. A., Parkinson, A. L., Eickhoff, C. R., Reetz, K., Hoffstaedter, F., . . . Eickhoff, S. B. (2015). The intrinsic resting state voice network in Parkinson's disease. Human Brain Mapping, 36(5), 1951-1962.
[https://doi.org/10.1002/hbm.22748]

-
Pinto, S., Ozsancak, C., Tripoliti, E., Thobois, S., Limousin-Dowsey, P., & Auzou, P. (2004). Treatments for dysarthria in Parkinson's disease. The Lancet Neurology, 3(9), 547-556.
[https://doi.org/10.1016/S1474-4422(04)00854-3]

-
Poline, J. B., Worsley, K. J., Evans, A. C., & Friston, K. J. (1997). Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage, 5(2), 83-96.
[https://doi.org/10.1006/nimg.1996.0248]

-
Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage, 62(2), 816-847.
[https://doi.org/10.1016/j.neuroimage.2012.04.062]

-
Ramig, L., Fox, C., & Sapir, S. (2011). Speech and voice disorders in Parkinson's disease. In C. W. Olanow, F. Stocchi, & A. E. Lang (Eds.) Parkinson's disease (pp. 346-360). Oxford: Blackwell Publishing Ltd.
[https://doi.org/10.1002/9781444397970.ch31]

-
Ramnani, N. (2006). The primate cortico-cerebellar system: Anatomy and function. Nature Reviews Neuroscience, 7(7), 511-522.
[https://doi.org/10.1038/nrn1953]

-
Riecker, A., Mathiak, K., Wildgruber, D., Erb, M., Hertrich, I., Grodd, W., & Ackermann, H. (2005). fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology, 64(4), 700-706.
[https://doi.org/10.1212/01.WNL.0000152156.90779.89]

-
Saad, Z. S., Glen, D. R., Chen, G., Beauchamp, M. S., Desai, R., & Cox, R. W. (2009). A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage, 44(3), 839-848.
[https://doi.org/10.1016/j.neuroimage.2008.09.037]

-
Simonyan, K., & Fuertinger, S. (2015). Speech networks at rest and in action: Interactions between functional brain networks controlling speech production. Journal of Neurophysiology, 113(7), 2967-2978.
[https://doi.org/10.1152/jn.00964.2014]

-
Skodda, S. (2011). Aspects of speech rate and regularity in Parkinson's disease. Journal of the Neurological Sciences, 310(1-2), 231-236.
[https://doi.org/10.1016/j.jns.2011.07.020]

-
Skodda, S., Flasskamp, A., & Schlegel, U. (2011). Instability of syllable repetition in Parkinson's disease-influence of levodopa and deep brain stimulation. Movement Disorders, 26(4), 728-730.
[https://doi.org/10.1002/mds.23439]

-
Skodda, S., Gronheit, W., & Schlegel, U. (2011). Intonation and speech rate in Parkinson's disease: General and dynamic aspects and responsiveness to levodopa admission. Journal of Voice, 25(4), e199-205.
[https://doi.org/10.1016/j.jvoice.2010.04.007]

-
Skodda, S., Visser, W., & Schlegel, U. (2010). Short- and long-term dopaminergic effects on dysarthria in early Parkinson's disease. Journal of Neural Transmission, 117(2), 197-205.
[https://doi.org/10.1007/s00702-009-0351-5]

-
Skodda, S., Visser, W., & Schlegel, U. (2011). Gender-related patterns of dysprosody in Parkinson disease and correlation between speech variables and motor symptoms. Journal of Voice, 25(1), 76-82.
[https://doi.org/10.1016/j.jvoice.2009.07.005]

-
Strick, P. L., Dum, R. P., & Fiez, J. A. (2009). Cerebellum and nonmotor function. Annual Review of Neuroscience, 32, 413-434.
[https://doi.org/10.1146/annurev.neuro.31.060407.125606]

-
Tahmasian, M., Eickhoff, S. B., Giehl, K., Schwartz, F., Herz, D. M., Drzezga, A., . . . Eickhoff, C. R. (2017). Resting-state functional reorganization in Parkinson's disease: An activation likelihood estimation meta-analysis. Cortex, 92, 119-138.
[https://doi.org/10.1016/j.cortex.2017.03.016]

-
van den Heuvel, M. P., & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519-534.
[https://doi.org/10.1016/j.euroneuro.2010.03.008]

-
Wang, Y. T., Kent, R. D., Duffy, J. R., Thomas, J. E., & Weismer, G. (2004). Alternating motion rate as an index of speech motor disorder in traumatic brain injury. Clinical Linguistics & Phonetics, 18(1), 57-84.
[https://doi.org/10.1080/02699200310001596160]

-
Wildgruber, D., Ackermann, H., & Grodd, W. (2001). Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: Effects of syllable repetition rate evaluated by fMRI. Neuroimage, 13(1), 101-109.
[https://doi.org/10.1006/nimg.2000.0672]

-
Wu, T., & Hallett, M. (2013). The cerebellum in Parkinson's disease. Brain, 136(3), 696-709.
[https://doi.org/10.1093/brain/aws360]

참 고 문 헌
- 강연욱, 박재설, 유경호, 이병철 (2009). 혈관성 인지장애 선별검사로서 Korean-Montreal Cognitive Assessement(K-MoCA)의 신뢰도, 타당도 및 규준 연구. 한국심리학회지: 임상, 28(2), 549-562.
- 김향희, 나덕렬 (1997). 한국판 보스턴 이름대기 검사. 서울: 학지사.
- 김향희, 나덕렬 (2012). 파라다이스 한국판 웨스턴 실어증검사 개정판. 서울: 파라다이스.
- 조맹제, 배재남, 서국희, 함봉진, 김장규, 이동우, 강민희 (1999). DSM-Ⅲ-R 주요우울증에 대한 한국어판 Geriatric Depression Scale(GDS)의 진단적 타당성 연구. 신경정신의학, 38(1), 48-63.