Research on Dementia in Communication Sciences and Disorders: Literature Analysis of Two Decades Using Text Mining
Copyright 2020 ⓒ Korean Speech-Language & Hearing Association.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
As an increasing number of people live with dementia, vast research on dementia is necessary. However, it is unclear how much dementia has been studied and in what context in the field of Communication Sciences and Disorders (CSD). This study was designed to explore the quantity of dementia research between 2000 and 2019 and CSD conditions studied in association with dementia using text mining techniques.
47,681 peer-reviewed articles published between the years 2009 and 2019 were extracted from the Web of Science, PsycINFO, and CINAHL. Text mining was carried out to understand productivity of research on dementia and other CSD conditions. Furthermore, co-occurrence network was analyzed to explore which dementia conditions were studied in relation with CSD conditions.
Fifty-three CSD conditions, including 10 different dementias, were identified. The number of studies on dementia was approximately half of that on language impairment or autism spectrum disorders. Among the 10 different dementias, unspecified dementia was most explored, followed by Alzheimer’s disease. Dementia studies have been increasing gradually in quantity but not significantly. Co-occurrence analysis revealed that primary progressive aphasia was the only dementia condition that showed a relatively stronger relation with other CSD conditions. Mild cognitive impairment and amyotrophic lateral sclerosis were also associated with dementia, but the association was not statistically significant.
Despite the importance of providing high-quality evidence specifically relevant to the field of CSD, dementia research has not been as rigorous as needed. Besides, dementia was found to be studied independently. Given the increasing aging population and a variety of impairments that people with dementia experience, it is promising to publish more studies focusing on dementia from more diverse perspectives.
Keywords:
Dementia, literature analysis, text mining, research trend, communication disordersⅠ. Introduction
As the world is aging, a growing need exists for clinical assessment and management of older people with cognitive impairment and dementia (Brookmeyer et al., 1998). According to the World Health Organization (WHO), the prevalence of people with dementia (PwD) is expected to increase to 22% of the entire population by 2050 (WHO, 2019). Consequently, problems in socioeconomic and health status due to increasing dementia prevalence have been reported (Bonin-Guillaume et al., 2005; Schneider et al., 1999). To cope with the problems associated with increasing dementia prevalence, researchers have shown efforts to identify different types of dementia, discover early signs of dementia, develop intervention approaches, design prevention strategies, compensatory strategies, and provide support for PwD and caregivers. As a result, the literature on dementia largely increased relative to the development of medical and psychiatry research across 36 years (from 1974 to 2009) (Theander & Gustafson, 2012). It was also found that dementia research has been most active in the field of psychiatry and psychology since the year of 2000 (Shen et al., 2019).
Meanwhile, the status of research on dementia in the specific field of communication sciences and disorders (CSD) is unclear. It is crucial to provide literature on dementia for Speech-Language Pathologists (SLPs) and Audiologists for two reasons: First, the scope of practice for SLPs and audiologists, the two major occupations affiliated with CSD, has been expanding with the increasing prevalence of dementia to provide clinical and educational services for PwD and their caregivers (American Speech-Language-Hearing Association, 2016, 2018; Bourgeois et al., 2016). Dementia is the fastest-growing caseload for SLPs (Bayles & Tomoeda, 2013). More specifically, PwD represents 15% of caseload for SLPs working in healthcare settings, and those in skilled nursing facilities reported 27% of their time is spent providing services for PwD (ASHA, 2019). Moreover, hearing loss is often found in PwD (Hubbard et al., 2018). Considering that it is more difficult for older adults with cognitive impairment to receive adequate audiological treatment when necessary profound knowledge about the challenges of aging as well as dementia is required for audiologists (Dupuis et al., 2013). Second, active investigations on dementia in CSD are essential given that they contribute to higher-level evidence of assessment and treatment efficacy. Assessment of communication deficits associated with dementia (e.g., memory loss, difficulty with comprehensive and/or expressive language, and hearing loss) is a concern among SLPs and audiologists working with PwD. Communicative deficits often occur not only in PwD but also in people with other communication disorders, and therefore have long been investigated by researchers in the CSD field. However, when it comes specifically to research on assessment of and interventions for deficits caused by or co-occur with dementia, it remains unclear to what extent and in what context dementia research has been conducted in the field of CSD.
Therefore, the current study was designed to explore the following questions using text mining techniques:
- 1. What is the study productivity of dementia in comparison to that of other communication disorders?
- 2. Which dementia conditions were studied in relation with other communication disorders?
Ⅱ. Methods
1. Identification of Studies
A systematic search of relevant databases was undertaken to locate journal articles that investigated communication disorders during the last decade. The databases searched for this study were Web of Science, PsycINFO, and CINAHL, in which research articles relevant to communication disorders were most likely to be published.
Searches were restricted to peer-reviewed journal papers in English published between 2000 and 2019. The limit resulted in excluding bibliographies of original articles, review articles, book chapters, opinion papers, and conference proceedings. A combination of terms that refer to communication disorders was used in an attempt to identify the most relevant papers. The incorporated terms were those commonly used to refer to CSD, such as disorder, impairment, and therapy. Example terms are “speech-language pathology,” “speech-language pathologists,” “speech pathology,” “speech therapy,” “language disorder,” and “language impairment.” In addition, truncation was used to include all possible morphological differences (i.e., speech-language pathology*, speech pathology*, speech impair*, speech disorder*, language disorder*, language impair*). These search terms were limited and combined using a Boolean operator ‘OR.’ The title and abstract of the identified articles from the search were analyzed in the current study.
2. Search Outcomes
A total of 47,681 journal articles were collected and analyzed from the databases after removing duplications. The initial search yielded a total of 64,312 articles which consisted of 35,008 hits from the Web of Science, 17,715 hits from PsycINFO, and 11,589 hits from CINAHL were yielded. Of those, 16,631 articles were found as duplications and dropped from the analysis.
3. Data Analysis
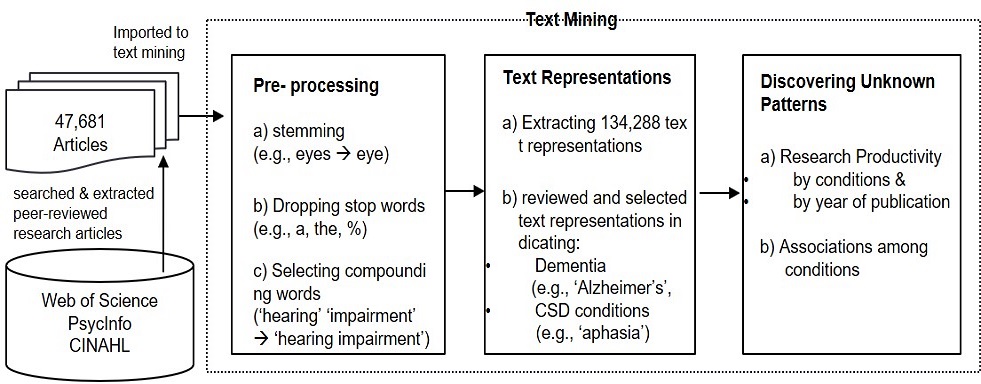
Text mining was carried out to extract relevant terms and trace relationships among dementia and other communication disorders examined in the published research. Text mining is a computer-supported process of extracting useful information and discovering latent patterns from unstructured textual data by utilizing various information retrieval, information extraction, and natural language processing techniques and algorithms (Hotho et al., 2005). This relatively new set of analytic techniques enables researchers to process scaled literature and facilitate the systematic and efficient reviews with analytic techniques such as identification of relevant studies, rapid description, categorization, summarization, and visualization (Malheiros et al., 2007; Thomas et al., 2011; O’Mara-Eves et al., 2015). A growing body of research using text mining techniques in a variety of research areas from health care and medicine (Abbe et al., 2016; Korhonen et al., 2012) to the economy (Abbas et al., 2014) and business (Moro et al., 2015). KH coder3 (Higuchi, 2018), an open text mining analysis software, used in the current review.
Three phases of analytics were conducted according to three general steps of text analytics: preprocessing, text representation, and knowledge discovery. In the first phase, the preprocessing phase was segmented into three steps: a) stemming which cuts the end of words to make it its original form (e.g., ‘says’ and ‘saying’ to ‘say,’), b) dropping stop words (e.g., ‘of’, ‘kg,’ and ‘%’) and generic terms (e.g., ‘abstract,’ person,’ and ‘general’), and c) extracting and selecting compound words. The compound terms were extracted by the researchers of this study through review on the extracted individual terms to derive more meaningful text representations. The researchers of this study also reviewed associated sentences to reflect the contextual meaning of individual compounding terms. For example, the term ‘quality of life’ was extracted as a single term, rather than extracting two terms ‘life’ and ‘quality.’ The reviewed and selected compounding terms were imported to the KH coder 3 software and extracted as single terms or text representations.
In the following phase, text representations were extracted, resulting in 134,288 terms. The researchers of this study independently reviewed and identified terms about dementia (e.g., ‘Alzheimer,’ ‘Lewy bodies,’ and ‘Huntington’) and other CSD conditions (e.g., ‘hearing impairment,’ ‘autism spectrum disorders,’ and ‘aphasia’). Synonyms, abbreviations, and substrings were merged as a single text representation. For example, for autism spectrum disorders, terms such as ‘ASD’ ‘autistic spectrum disorder,’ and ‘autistic spectrum’ were grouped as a single term in the analysis. To select the CSD related terms, we referenced Communication Disorders Taxonomy (Government of Malta, 2019). Medical Subject Headings (MeSH) 2020 (U.S. National Library of Medicine, 2020) and its tree structures were also referenced to group dementia. The disagreement was solved by discussion. Hierarchical relationships among the identified terms were ignored because text mining analyses are based on text representation such as term, it is regarded as an appearance of the particular concept in a dataset. This study, accordingly, assumed that generic condition name such as dementia was found, we regarded it as a representation of the research topic or subjects in the collected publication. For example, dementia can include Alzheimer’s disease (AD), Huntington’s diseases (HD), Lewy Bodies dementia (LBD) and others, but in case of studies did not specify what type(s) of dementia they examined, we simply extracted and used the term ‘dementia’ in the analyses. The selection resulted in 43 terms regarding CSD conditions, ten terms of dementia. Appendix A provides the list of the terms included in the analyses.
Once term selection was completed, research productivity by conditions and by year of publication, and the relationships among studied conditions were computed to answer research questions about the current investigation. The following techniques and indexes were employed: Document frequency (df) was used to compute the productivity of research by conditions in total. Document frequency refers to the number of unique published papers containing individual terms. In the current study, df indicates how many unique studies were conducted regarding individual conditions. The percentage and number of articles to identify dementia and other CSD conditions concerning the year of publication were also computed using the crosstab command embedded in the software. Finally, co-occurrence network analysis was applied to discover and visually present the associations among dementia and other CSD conditions, which answers the research question (2). Co-occurrence, a network analysis technique, represents the association among the terms, assessing how frequently the paired terms co-occur in the same document (Darkes and Goldman, 1998; Wachs-Lopes and Rodrigues, 2016). Among different network analysis techniques, this study adopted modularity, which represents a network property to detect sub-group or division of that network into the community (Clauset et al., 2004). The edge of the network, or the degree of associations between paired terms in a network, was evaluated using the cosine coefficient. The index calculates co-occurrence patterns based on normalized frequency rather than raw frequency, which prevents the length of the document from affecting the evaluation. The score ranges from -1 meaning maximum dissimilarity to 1 indicating maximum similarity, and 0 meaning decorrelation. Figure 1 shows the analytic pipeline.
Ⅲ. Results
1. Studies on Dementia in Comparisom to Other CSD Conditions
A total of 53 conditions, which consisted of 43 CSD conditions excluding dementia and ten types of dementia, were identified in the dataset. Table 1 shows the top 20 most frequently found conditions. Among the other CSD conditions, language impairment was found most commonly, appearing in slightly over 5,900 (12.38% out of 47,681, df=5,908) journal articles. It was closely followed by autism. When considering language impairment is an umbrella term describing disorders of language that interfere with communication such as aphasia, it can be concluded that autism found in over 5,400 articles (11.52%, df=5,492), is the most commonly studied CSD condition. Of those identified conditions, dementia ranked fourth. This rank appeared relatively high. However, the quantity of related studies was only approximately half of those about language impairment or autistic spectrum disorder (ASD). Besides, the number of articles regarding dementia in Table 1, indicates the total amount of publications of ten types of detected dementia. As shown in Table 2, the percentages of publications on individual dementia conditions were 4% or lower, which were significantly smaller than other CSD conditions. Refer to Appendix A for detailed information about CSD conditions found in the dataset.

Top 20 most frequently identified terms related to communication sciences and disorders conditions between 2000 and 2019

Terms regarding individual dementia that were identified communication sciences and disorders studies between 2000 and 2019
Among different types of dementia, broader dementia without a specified cause (unspecified dementia hereafter) was found most common in the dataset (see Table 2). Despite its comparatively higher number of articles, the proportion of studies regarding general dementia is less than 4% (df=1,700, 3.57% out of 47,681). It was followed by AD (2.85%). The proportions of studies regarding other types of dementia was found to be less than 1%, which is less than one-third of unspecified dementia and AD studies. To illustrate, studies pertaining to frontotemporal dementia (FTD) and primary progressive aphasia (PPA) were only 0.98% (df=465) and 0.92% (df=440) respectively. Particularly, three types of dementia have been significantly understudied: only 33 studies (0.07%) appeared to concern neurofibrillary tangles, 16 studies were about Creutzfeldt-Jakob dementia (0.03%), and four studies regarded Klüver-Bucy syndrome (0.01%).
2. Study Productivity: Dementia and Other CSD Conditions by Year of Publication
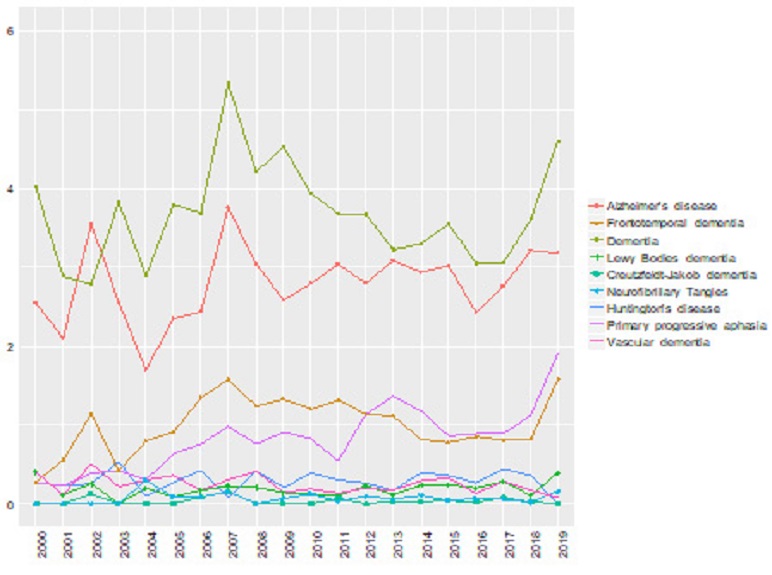
Examination on the publication productivity by year revealed that research into ASD has increased gradually and has become the most popular study subject since 2010. To illustrate, in 2000 only 4.56% (n=34) out of the 746 CSD publications concerned ASD. This proportion of studies on ASD increased to over 10% in 2010 and reached slightly less than 15% in 2018. Contrastingly, studies on aphasia, specific language impairment (SLI), and stuttering gained attention by researchers in the field of CSD before 2010 and the percentages of regarded studies have gradually reduced. The proportion of studies regarding unspecified language impairment had been consistently over 10% during the two decades. However, in comparison to over 17% of studies published in 2000, 12% of CSD research was about unspecified language impairment in 2019. The proportions of SLI and stuttering studies showed similar patterns, but the number of studies regarding these CSD conditions had been much less than that of unspecified language impairment, having been reduced by slightly over 5% to 1%. In other words, in the early 21st century, a relatively large portion of CSD studies focused on general language impairment issues, but recently the focus of CSD researchers had moved onto autism issues. Figure 2 visually shows the changes in the study proportions of the detected CSD conditions including dementia. See Table 3 for details of the top ten most frequently studied CSD conditions.
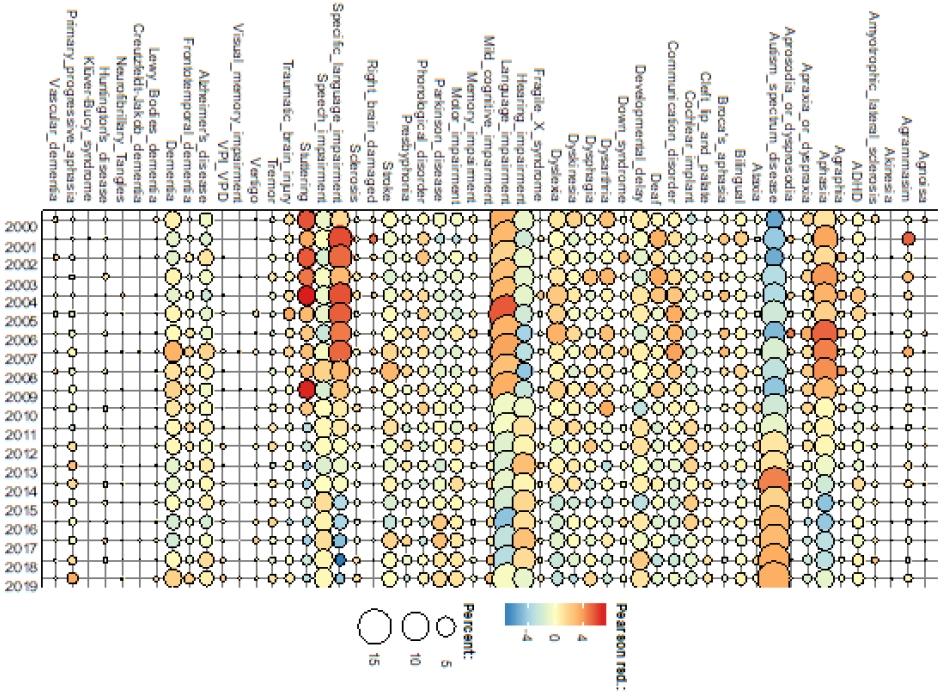
A bubble map of the identified dementia and other conditions in communication sciences and disorders by year

Studies productivity of top ten most frequently identified dementia and other communication sciences and disorders conditions by year
The productivity of studies on dementia had gradually increased but not significantly. When combined with all types of dementia included in this study, only 61 studies (8.18%) out of 746 articles in 2000 studied dementia. The quantity and proportion of studies on dementia had grown to 149 (10.3% out of 1,447) in 2008, 293 (9.53% out of 3,076) in 2012, and 454 (9.42% out of 4,820) in 2018 (see Table 3). These proportions and the number of studies were still relatively smaller than those of other popularly studied CSD conditions such as language impairment or ASD. When examining individual dementia conditions, the proportions of studies about unspecified dementia and AD have been consistently higher than other types of dementia across the last two decades (see Figure 2). The proportion of unspecified dementia studies have ranged from approximately three to four percent (see Figure 2 and Table 3). Studies regarding AD had ranged from slightly over two to three percent. Despite the relatively consistent proportions of studies regarding these two conditions, the quantities of studies had been increased. To illustrate, the number of AD studies had increased from 19 to 155, and unspecified dementia studies have increased from 22 to 174 during the last two decades (see Table 4). Aside from AD and unspecified dementia, the number of studies regarding PPA and FTD recently started to increase as shown in Figure 3.
3. Associations Among Dementia and Other CSD Conditions in Research
To discover which CSD conditions shared their areas with dementia or vice versa, co-occurrence analysis was conducted. Figure 4 visually represents the associations among the sub-group structure of dementia and other CSD conditions. Conditions in a sub-group, which were associated with lines and presented in the same color, indicates a significant amount of research regarded the paired conditions in the same study. A total of seven groups of CSD conditions were detected. Overall, the group of dementia and other CSD conditions appeared to have weak relationships in the network. The group with ASD, language impairment, and aphasia were found to consist of the largest groups of researched conditions. A majority of dementia conditions, which highlighted in yellow in Figure 4, were grouped separately from other CSD conditions. This finding implies that major study topics, such as ASD, language impairment, and aphasia, in the field of CSD had not actively involved dementia.
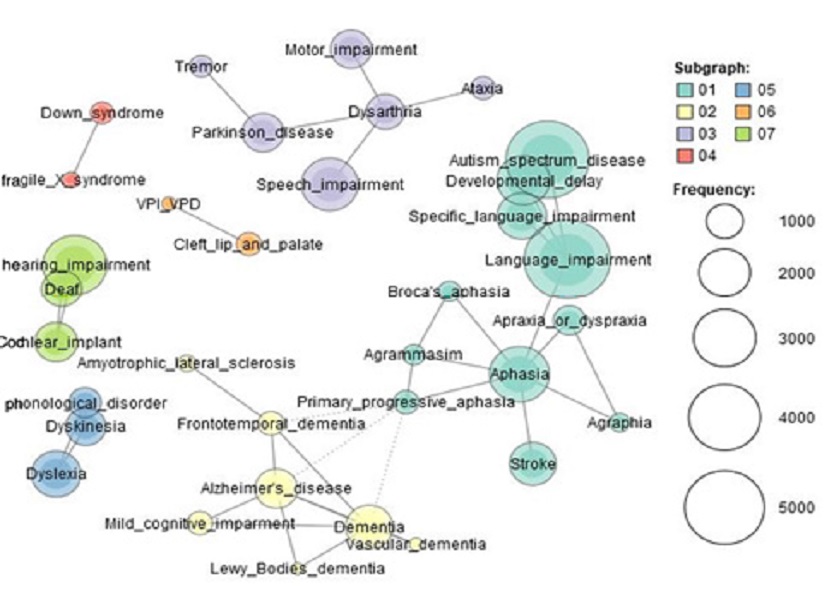
Co-occurrence network of conditions involved in communication sciences and disorders based on modularity (node=34, edge=40, D=.071)
Only a few specific CSD conditions were discussed in connection with dementia. PPA was found to be the only dementia condition that was associated with the dementia group, showing relatively stronger associations with AD, unspecified dementia, and frontotemporal dementia. This result refers that unlike other dementia conditions PPA is relatively often studied in relation with CSD conditions. At the same time, PPA is also more frequently addressed with the three dementia studies. Other CSD conditions that appeared to have been closely discussed regarding dementia were amyotrophic lateral sclerosis (ALS) and mild cognitive impairment (MCI). Although studies regarding these two conditions were not significant in amount, these conditions were closely discussed in connection with dementia in the field of CSD.
Ⅳ. Discussion
The overarching aim of this study was to investigate the current status of dementia research in the field of CSD by using text mining techniques. Specifically, the first purposes of this study was to investigate the quantity of dementia research compared to research on other CSD conditions. Although a relative scarcity of dementia research compared to other CSD conditions such as ASD and aphasia, CSD researchers have started to pay more attention to dementia. This trend is found in other areas of study such as medicine and psychiatry (Theander & Gustafason, 2015), gerontology (Shen et al., 2019), and nursing (Roh, 2008). Among all dementia types, AD comprised the largest portion of dementia research, when excluding research that unspecified types of dementia were studied. This is not surprising given that AD is the leading cause of dementia that accounts for 60 to 80 percent of all dementia cases (Alzheimer’s Association, n.d.). It is vital to continue to increase the amount of high-quality research on dementia in the field of CSD given that a considerable number of SLPs and audiologists are currently working with PwD, with the caseload expected to increase.
The second purpose of the current investigation was to explore associations between dementia and other CSD conditions in research. Interestingly, dementia appeared to be studied in a separate context. PPA was the only dementia condition that was frequently associated with a particular group of communication disorders – aphasia. The strong association between PPA and aphasia in CSD research may be due to the overlapping characteristics of dementia and aphasia that people with PPA experience. Since Pick (1892) first described a language disorder associated with atrophy of the frontal and temporal regions in the dominant hemisphere, researchers have renamed and/or recategorized the disorder to better capture its underlying symptoms: slowly progressive aphasia then primary progressive aphasia by Mesulam (1982); semantic dementia by Snowden et al. (1989) and, progressive nonfluent aphasia by Grossman et al. (1996). This implies that researchers were unsure of this disorder’s category – is this aphasia or dementia? Finally, a group of researchers attempted to build concrete criteria (Neary et al., 1998) but the category of PPA remained unclear. Some categorized it as semantic dementia or progressive nonfluent aphasia, and there is another variant, logopenic progressive aphasia, a relatively new variant described and defined by Gorno-Tempini et al. (2004). Although typical features of PPA have not been reached to a clear consensus (Gorno-Tempini et al., 2004), PPA nowadays is considered a type of dementia, FTD in particular, given heterogeneous biomarkers (e.g., TDP-43) and its progressive language and behavioral impairments that are more par for the course of dementia rather than aphasia. However, for a person to meet the criteria of PPA, the person should show aphasic deficits at the time of examination (Mesulam et al., 2009). Other than PPA, ALS and MCI were also found to have an association with dementia, although non-significant. This is not startling because MCI increases the risk of developing dementia (Luck et al., 2012; Manly et al., 2008; Ravaglia et al., 2008). Similarly, clinical studies of cognitive and behavioral impairments in ALS showed that about 20% of people with ALS exhibit dementia, and 30% of them have cognitive impairments that are not dementia (Lomen-Hoerth et al., 2003; Murphy et al., 2007).
Despite the increasing number of publications on dementia in CSD, dementia was found to have no significant association with speech, language, or hearing impairments. Further research needs to be directed towards expanding knowledge in dementia, specifically applicable in the field of CSD. Although the majority of studies, based on the search for the current investigation, focused on AD, other types of dementia should also be investigated given that different types of dementia are associated with different areas of speech and/or language impairments. However, different types of dementia affect human speech and language (Braaten et al., 2006) in divergent ways. Therefore, investigating the speech and language characteristics of different types of dementia may be an early or a differential diagnostic factor as seen in Forbes et al. (2002), Grossman & Ash (2004), and Abdalla et al. (2017). Given this finding, there have been investigations on the detection of speech and language impairments in PwD. To briefly summarize, studies on speech and language impairments found following symptoms associated with a specific type of dementia: AD with word-level impairments such as word-finding, naming, and word comprehension; vascular dementia with those found in AD as well as incomprehensible speech and decreased complexity; Lewy body disease with language disorders, including the symptoms of AD and Parkinson disease dementia (PDD); PDD with non-articulate, slow, and non-grammatic speech and decreased verbal fluency; PPA with slow and hesitating speech, worsening understanding of complex speech, loss reading and writing; semantic dementia with representatively a lack of vocabulary (Klimova & Kuca, 2016). In addition, people with AD commonly show apraxia of speech (Chandra et al., 2015), and dysarthria is common in PDD (Klimova & Kuca, 2016). Recently, researchers have focused on machine learning to identify the speech and language impairments associated with a specific type of dementia more accurately and efficiently (Balagopalan et al., 2018; Fraser et al., 2016; Haider et al., 2019; Hernandez-Dominguez et al., 2018; Mirheidari et al., 2018; Orimaye et al., 2017; Pompili et al., 2020; Tanaka et al., 2017; Zargarbashi, & Babaali, 2019; Zhigiang et al., 2019; Zhu et al., 2019).
Not only does PwD show speech and language impairments, but they also experience hearing impairments. It is noteworthy that dementia was found not to co-occur with hearing impairment in CSD research despite the high prevalence of age-related hearing impairment: 25% of people between ages 60 and 69, 55% of those aged 70 to 79, and approximately 80% of those older than 80 (Lin et al., 2011). Hearing loss is related to incident dementia, regardless of the independent types (Kim et al., 2018; Lin et al., 2011; Uhlmann et al., 1989). More significantly, hearing loss expedites cognitive decline in PwD (Gurgel et al., 2014) and hearing aids do not prevent deterioration in cognitive functioning (Allen, 2003). Thus, CSD scholars must disseminate more knowledge of dementia and hearing loss relevant to clinical and educational services for PwD and their caregivers, as stated in audiologists’ scope of practice defined by ASHA (ASHA, 2018).
Some limitations of the investigation should be addressed in future studies. First, the current study explored a broad set of research questions to provide a birds-eye view of state-of-the-art dementia research in CSD. Future studies using text mining techniques to answer more specific research questions that contribute to theoretical and clinical advances are warranted. Second, the underlying reasons for the weak relationship between dementia and speech, language, and/or hearing impairment were not explored in this study. It may be attributed to the limited search period (2000~2019), or limited clinical research on language, communication impairments in dementia, especially non-AD types, as Ferris and Farlow (2013) addressed. Studies investigating what factors shape the weak relationship between dementia and other CSD conditions may be beneficial for building a bridge between the two parties and ultimately for better-incorporating dementia into the field of CSD.
In sum, this study presents a relatively novel approach to quantitatively demonstrate the current state of dementia research in CSD. Based on the findings of the study, it is encouraged to produce more publications on dementia in CSD to expand knowledge about dementia from the perspectives of communication science and clinical contexts for SLPs and audiologists.
Reference
-
Abbas, A., Zhang, L., & Khan, S. U. (2014). A literature review on the state-of-the-art in patent analysis. World Patent Information, 37, 3–13.
[https://doi.org/10.1016/j.wpi.2013.12.006]

- Abbe, A., Grouin, C., Zweigenbaum, P., & Falissard, B. (2016). Text mining applications in psychiatry: A systematic literature review. International Journal of Methods in Psychiatric Research, 25(2), 86–100.
-
Abdalla, M., Rudzicz, F., & Hirst, G. (2017). Rhetorical structure and Alzheimer’s disease. Aphasiology, 32(1), 41–60.
[https://doi.org/10.1080/02687038.2017.1355439]

-
Allen, N. H. (2003). The effects of improving hearing in dementia. Age and Ageing, 32(2), 189-193.
[https://doi.org/10.1093/ageing/32.2.189]

- Alzheimer’s Association. (n.d.). What is dementia? Retrieved from https://www.alz.org/alzheimers-dementia/what-is-alzheimers
- American Speech-Language-Hearing Association. (2016). Scope of practice in speech‑language pathology [Scope of Practice]. Retrieved from www.asha.org/policy
- American Speech-Language-Hearing Association. (2018). Scope of practice in audiology [Scope of Practice]. Retrieved from www.asha.org/policy
- American Speech-Language-Hearing Association. (2019). ASHA 2019 SLP health care survey: Caseload characteristics. Retrieved from https://www.asha.org/uploadedFiles/2019-SLP-HC-Survey-Caseload.pdf
- Balagopalan, A., Novikova, J., Rudzicz, F., & Ghassemi, M. (2018). The effect of heterogeneous data for Alzheimer's disease detection from speech. arXiv:1811.12254, [cs.LG]
- Bayles, K. A., & Tomoeda, C. K. (2013). Cognitive-communication disorders of dementia: Definition, diagnosis, and treatment (2nd ed.). San Diego: Plural Publishing, Inc.
-
Bonin-Guillaume, S., Zekry, D., Giacobini, E., Gold, G., & Michel, J. P. (2005). The economical impact of dementia. Presse Medicale, 34(1), 35–41.
[https://doi.org/10.1016/S0755-4982(05)83882-5]

-
Bourgeois, M., Brush, J., Douglas, N., Khayum, R., & Rogalski, E. (2016). Will you still need me when I'm 64, or 84, or 104? The importance of speech-language pathologists in promoting the quality of life of aging adults in the united states into the future. Seminars in Speech and Language, 37(3), 185–200.
[https://doi.org/10.1055/s-0036-1583544]

-
Braaten, A. J., Parsons, T. D., McCue, R., Sellers, A., & Burns, W. J. (2006). Neurocognitive differential diagnosis of dementing diseases: Alzheimer's dementia, vascular dementia, frontotemporal dementia, and major depressive disorder. The International Journal of Neuroscience, 116(11), 1271–1293.
[https://doi.org/10.1080/00207450600920928]

-
Chandra, S., Issac, T., & Abbas, M. (2015). Apraxias in neurodegenerative dementias. Indian Journal of Psychological Medicine, 37(1), 42.
[https://doi.org/10.4103/0253-7176.150817]

-
Clauset, A., Newman, M. E. J., & Moore, C. (2004). Finding community structure in very large networks. Physical Review E, 70(6).
[https://doi.org/10.1103/PhysRevE.70.066111]

-
Darkes, J., & Goldman, M. S. (1998). Expectancy challenge and drinking reduction: Process and structure in the alcohol expectancy network. Experimental and Clinical Psychopharmacology, 6(1), 64–76.
[https://doi.org/10.1037/1064-1297.6.1.64]

-
Dupuis, K., Reed, M., Lemke, U., & Pichora-Fuller, M. (2013). Helping older people with cognitive decline communicate: Hearing aids as part of a broader rehabilitation approach. Seminars in Hearing, 34(04), 308–330.
[https://doi.org/10.1055/s-0033-1356643]

-
Ferris, S. H., & Farlow, M. (2013). Language impairment in Alzheimer's disease and benefits of acetylcholinesterase inhibitors. Clinical Interventions in Aging, 8, 1007–1014.
[https://doi.org/10.2147/CIA.S39959]

-
Forbes, K. E., Venneri, A., & Shanks, M. F. (2002). Distinct patterns of spontaneous speech deterioration: An early predictor of Alzheimer's disease. Brain and Cognition, 48(2-3), 356–361.
[https://doi.org/10.1006/brcg.2001.1377]

-
Fraser, K. C., Meltzer, J. A., & Rudzicz, F. (2016). Linguistic features identify Alzheimer's disease in narrative speech. Journal of Alzheimer's Disease, 49(2), 407–422.
[https://doi.org/10.3233/JAD-150520]

- Gorno-Tempini, M. L., Dronkers, N. F., Rankin, K. P., Ogar, J. M., Phengrasamy, L., Rosen, H. J., . . . Miller, B. L. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346.
- Government of Malta. (2019). SLP Conditions. Retrieved from https://deputyprimeminister.gov.mt/en/sls/Pages/Conditions-which-may-affect-Speech-and-Language.aspx
-
Grossman, M., & Ash, S. (2004). Primary progressive aphasia: A review. Neurocase, 10(1), 3-18.
[https://doi.org/10.1080/13554790490960440]

-
Grossman, M., Mickanin, J., Onishi, K., Hughes, E., D’Esposito, M., Ding, X.-S., . . . Reivich, M. (1996). Progressive nonfluent aphasia: Language, cognitive, and pet measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience, 8(2), 135–154.
[https://doi.org/10.1162/jocn.1996.8.2.135]

-
Gurgel, R. K., Ward, P. D., Schwartz, S., Norton, M. C., Foster, N. L., & Tschanz, J. T. (2014). Relationship of hearing loss and dementia. Otology & Neurotology, 35(5), 775–781.
[https://doi.org/10.1097/MAO.0000000000000313]

-
Haider, F., de la Fuente, S., & Luz, S. (2020). An assessment of paralinguistic acoustic features for detection of Alzheimer’s dementia in spontaneous speech. IEEE Journal of Selected Topics in Signal Processing, 14(2), 272–281.
[https://doi.org/10.1109/JSTSP.2019.2955022]

-
Hubbard, H. I., Mamo, S. K., & Hopper, T. (2018). Dementia and hearing loss: Interrelationships and treatment considerations. Seminars in Speech and Language, 39(3), 197-210.
[https://doi.org/10.1055/s-0038-1660779]

-
Hernández-Domínguez, L., Ratté, S., Sierra-Martínez, G., & Roche-Bergua, A. (2018). Computer-based evaluation of Alzheimer's disease and mild cognitive impairment patients during a picture description task. Alzheimer's & Dementia, 10(Amst), 260–268.
[https://doi.org/10.1016/j.dadm.2018.02.004]

- Higuchi, K. (2018). KH coder. Retrieved from http://khcoder.net/en/
- Hotho, A., Nürnberger, A., & Paaß, G. (2005). A brief survey of text mining. Ldv Forum, 20(1), 19-62.
-
Kim, S. Y., Lim, J.-S., Kong, I. G., & Choi, H. G. (2018). Hearing impairment and the risk of neurodegenerative dementia: A longitudinal follow-up study using a national sample cohort. Scientific Reports, 8(1).
[https://doi.org/10.1038/s41598-018-33325-x]

-
Klimova, B., & Kuca, K. (2016). Speech and language impairments in dementia. Journal of Applied Biomedicine, 14(2), 97–103.
[https://doi.org/10.1016/j.jab.2016.02.002]

-
Korhonen, A., Séaghdha, D. Ó., Silins, I., Sun, L., Högberg, J., & Stenius, U. (2012). Text mining for literature review and knowledge discovery in cancer risk assessment and research. PLoS ONE, 7(4).
[https://doi.org/10.1371/journal.pone.0033427]

-
Lin, F. R., Metter, E. J., O’Brien, R. J., Resnick, S. M., Zonderman, A. B., & Ferrucci, L. (2011). Hearing loss and incident dementia. Archives of Neurology, 68(2), 1546-1554.
[https://doi.org/10.1001/archneurol.2010.362]

-
Lin, F. R., Thorpe, R., Gordon-Salant, S., & Ferrucci, L. (2011). Hearing loss prevalence and risk factors among older adults in the united states. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 66A(5), 582–590.
[https://doi.org/10.1093/gerona/glr002]

-
Lomen-Hoerth, C., Murphy, J., Langmore, S., Kramer, J. H., Olney, R. K., & Miller, B. (2003). Are amyotrophic lateral sclerosis patients cognitively normal? Neurology, 60(7), 1094–1097.
[https://doi.org/10.1212/01.WNL.0000055861.95202.8D]

-
Luck, T., Luppa, M., Wiese, B., Maier, W., Van den Bussche, H., Eisele, M., . . . Riedel-Heller, S. G. (2012). Prediction of incident dementia: Impact of impairment in instrumental activities of daily living and mild cognitive impairment—Results from the german study on ageing, cognition, and dementia in primary care patients. The American Journal of Geriatric Psychiatry, 20(11), 943–954.
[https://doi.org/10.1097/JGP.0b013e31825c09bc]

-
Malheiros, V., Hohn, E., Pinho, R., Mendonca, M., & Maldonado, J. C. (2007). A visual text mining approach for systematic reviews. Proceedings of First International Symposium on Empirical Software Engineering and Measurement (ESEM 2007), Madrid, 245-154.
[https://doi.org/10.1109/ESEM.2007.21]

-
Manly, J. J., Tang, M.-X., Schupf, N., Stern, Y., Vonsattel, J.-P. G., & Mayeux, R. (2008). Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology, 63(4), 494–506.
[https://doi.org/10.1002/ana.21326]

-
Mesulam, M.-M. (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598.
[https://doi.org/10.1002/ana.410110607]

-
Mesulam, M., Wieneke, C., Rogalski, E., Cobia, D., Thompson, C., & Weintraub, S. (2009). Quantitative template for subtyping primary progressive aphasia. Archives of Neurology, 66(12).
[https://doi.org/10.1001/archneurol.2009.288]

-
Mirheidari, B., Blackburn, D., Walker, T., Venneri, A., Reuber, M., & Christensen, H. (2018). Detecting signs of dementia using word vector representations. INTERSPEECH, 2018, 1893-1897.
[https://doi.org/10.21437/Interspeech.2018-1764]

-
Moro, S., Cortez, P., & Rita, P. (2015). Business intelligence in banking: A literature analysis from 2002 to 2013 using text mining and latent Dirichlet allocation. Expert Systems with Applications, 42(3), 1314–1324.
[https://doi.org/10.1016/j.eswa.2014.09.024]

- Murphy, J., Henry, R., & Lomen-Hoerth, C. (2007). Establishing subtypes of the continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Archives of Neurology, 64(3), 330.
-
Neary, D., Snowden, J. S., Gustafson, L., Passant, U., Stuss, D., Black, S., . . . Benson, D. F. (1998). Frontotemporal lobar degeneration: A consensus on cLomen-Hoerthlinical diagnostic criteria. Neurology, 51(6), 1546–1554.
[https://doi.org/10.1212/WNL.51.6.1546]

-
O’Mara-Eves, A., Thomas, J., McNaught, J., Miwa, M., & Ananiadou, S. (2015). Using text mining for study identification in systematic reviews: A systematic review of current approaches. Systematic Reviews, 4(1), 5.
[https://doi.org/10.1186/2046-4053-4-5]

-
Orimaye, S. O., Wong, J. S., Golden, K. J., Wong, C. P., & Soyiri, I. N. (2017). Predicting probable Alzheimer's disease using linguistic deficits and biomarkers. BMC Bioinformatics, 18(1), 34.
[https://doi.org/10.1186/s12859-016-1456-0]

-
Pompili, A., Abad, A., de Matos, D. M., & Martins, I. P. (2020). Pragmatic aspects of discourse production for the automatic identification of Alzheimer’s Disease. IEEE Journal of Selected Topics in Signal Processing, 14(2), 261–271.
[https://doi.org/10.1109/JSTSP.2020.2967879]

-
Ravaglia, G., Forti, P., Montesi, F., Lucicesare, A., Pisacane, N., Rietti, E., . . . Mecocci, P. (2008). Mild cognitive impairment: Epidemiology and dementia risk in an elderly Italian population. Journal of the American Geriatrics Society, 56(1), 51–58.
[https://doi.org/10.1111/j.1532-5415.2007.01503.x]

- Roh, K. (2008). A review of the trends of journal research on dementia and nursing interventions for demented elders. Journal of Korean Academy of Community Health Nursing, 19(2), 300-309.
-
Schneider, J., Murray, J., Banerjee, S., & Mann, A. (1999). EUROCARE: A cross‐national study of co‐resident spouse carers for people with Alzheimer's disease: I—factors associated with carer burden. International Journal of Geriatric Psychiatry, 14(8), 651-661.
[https://doi.org/10.1002/(SICI)1099-1166(199908)14:8<651::AID-GPS992>3.0.CO;2-B]

-
Shen, C., Nguyen, D. T., & Hsu, P.-Y. (2019). Bibliometric networks and analytics on gerontology research. Library Hi Tech, 37(1), 88–100.
[https://doi.org/10.1108/LHT-11-2017-0247]

- Snowden, J. S., Goulding, P. J., & Neary, D. (1989). Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology, 2(3), 167-182.
-
Tanaka, H., Adachi, H., Ukita, N., Ikeda, M., Kazui, H., Kudo, T., & Nakamura, S. (2017). Detecting dementia through interactive computer avatars. IEEE Journal of Translational Engineering in Health and Medicine, 5, 1-11.
[https://doi.org/10.1109/JTEHM.2017.2752152]

-
Theander, S. S., & Gustafson, L. (2012). Publications on dementia in medline 1974-2009: A quantitative bibliometric study. International Journal of Geriatric Psychiatry, 28(5), 471–478.
[https://doi.org/10.1002/gps.3848]

-
Thomas, J., McNaught, J., & Ananiadou, S. (2011). Applications of text mining within systematic reviews. Research Synthesis Methods, 2(1), 1–14.
[https://doi.org/10.1002/jrsm.27]

- U.S. National Library of Medicine. (2020). MeSH subject headings 2020. Retrieved from https://meshb.nlm.nih.gov
-
Uhlmann, R. F., Larson, E. B., Rees, T. S., Koepsell, T. D., & Duckert, L. G. (1989). Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. The Journal of American Medical Association, 261(13), 1916–1919.
[https://doi.org/10.1001/jama.1989.03420130084028]

-
Wachs-Lopes, G. A., & Rodrigues, P. S. (2016). Analyzing natural human language from the point of view of dynamic of a complex network. Expert Systems with Applications, 45(1), 8–22.
[https://doi.org/10.1016/j.eswa.2015.09.020]

- Zargarbashi, S. H., & BabaAli, B. (2019). A multi-modal feature embedding approach to diagnose Alzheimer disease from spoken language. arXiv:1910.00330, [cs.LG]
-
Zhu, Z., Novikova, J., & Rudzicz, F. (2019). Detecting cognitive impairments by agreeing on interpretations of linguistic features. arXiv:1808.06570, [cs.CL]
[https://doi.org/10.18653/v1/N19-1146]