Effects of Chair-Based Fidgeting and Age on Prefrontal Brain Activity and Cognitive Performance
Copyright 2023 ⓒ Korean Speech-Language & Hearing Association.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The objective of this study was to evaluate the effects of chair-based fidgeting and age on prefrontal brain activities and cognitive performances of neurocognitive tasks involving executive functioning and working memory.
In a repeated-measures laboratory setting, 12 healthy adults (6 young and 6 older adults) were exposed to the chair-based fidgeting and the conventional sitting workstation, respectively. After the short-term exposure of each condition, participants performed five neurocognitive tasks oriented for executive functioning and working memory.
The hemodynamic activities via functional near- infrared spectroscopy (fNIRS) in the prefrontal cortex were significantly affected by the fidgeting conditions. The effects of the fidgeting condition on prefrontal brain activities depended on the age group. With the fidgeting intervention, older adults revealed increased brain activation in the prefrontal cortex compared to no fidgeting condition, whereas younger adults showed the opposite pattern. There were no significant differences in cognitive performance due to fidgeting.
The results indicate that chair-based fidgeting may increase the prefrontal brain activation of older adults without negatively affecting cognitive performances compared to the conventional sitting workstation. In other words, additional fidgeting activity did not disturb the sedentary work but induced more engagement in a brain activity. This intervention may be beneficial in preventing or improving cognitive deficits by alternating and involving more dynamic prefrontal brain activity. Therefore, this research may provide a starting point to develop better cognitive treatment approaches to support people with speech or language related cognitive deficits.
Keywords:
Chair-based fidgeting, fidgeting, fNIR, brain activity, cognitive performanceⅠ. Introduction
Office workers suffer from prolonged sitting, and the majority of the sitting duration was related to the computer-based nature of work (Brown et al., 2003). The traditional office designs also force office workers to remain seated throughout the day. Previous studies have been shown that sedentary behavior of office work was associated with various adverse health outcomes such as chronic disease, impaired cognition (Falck et al., 2016), obesity (Hamilton et al., 2007), and musculoskeletal disorders (Andersen et al., 2011). Although the mechanisms by which sitting behavior severely causes diseases are not well understood, the intermittent breaks have been shown a beneficial impact on the cardiometabolic risk markers (e.g., improvement in insulin sensitivity and lipids) (Dunstan et al., 2012).
In order to make sedentary work more dynamic, the efficacy of various intervention methods, including sit-stand desks, walking while working, and leaning workstations, has been investigated (Buckley et al., 2013; Kuppam et al., 2019; Le & Marras, 2016; Noma et al., 2004). It has been found that these interventions could reduce sitting time and improve posture, productivity, and cognitive function, although the longer-term health effects have not been proven yet. Despite the promising results of standing or walking while working interventions, these options may not be practical for many office workers (Júdice et al., 2015). Thus, alternative interventions could also be considered to enable sedentary workers to move more without a disturbance to their works.
The dynamic sitting intervention has recently gained great attention as a way to promote sedentary workers’ movements (Mörl & Bradl, 2013). There are several commercially-available office furniture that encourages office workers to adopt fidgeting movements while working at their regular jobs (Pynt, 2015). Previous studies showed that chairs and devices promoting fidgeting or leg movements increased energy expenditure by 20% relative to the conventional sitting work (Koepp et al., 2016; Levine et al., 2000). However, there is a lack of studies on how these devices could benefit sedentary workers’ brain activities and cognitive function.
The exercise is known to be positively associated with the blood flow to the brain and the cognitive functions, including executive function and working memory (Davis et al., 2011). Executive function is regarded as the group of cognitive processes directing human behaviors (e.g., inhibitions, goal planning, and selective attention) (Miyake et al., 2000). A previous study showed that children’s executive function was improved by aerobic exercise (both acute and chronic) (Best, 2010). Working memory is the temporary storage or the process of manipulation of the information in the brain (D’Esposito et al., 1995). It was found that the response speed of working memory tasks was improved by the intermediate intensity exercise (McMorris et al., 2011). The associations between the frontal brain activities and executive function and working memory were found from previous neuroimaging studies (Carpenter, 2000). Therefore, increasing energy expenditure with the aid of dynamic sitting may improve cognitive function (e.g., executive function and working memory). An fNIR (functional Near-Infrared Spectroscopy) is a non-invasive brain imaging technique measuring blood oxygenation changes. When a brain becomes active, the change of oxygen in the blood is expected. This approach has been widely applied to assess cognition and motor control (Saraiv et al., 2021).
The executive function was found to be affected by age. There were varying degrees from mild to severe age-related decline in executive function (Bryan and Luszcz, 2000). The working memory was also influenced by age. A previous study found a negative correlation between age and working memory, which was associated with the storage capacity, processing efficiency and coordination effectiveness (Salthouse & Babcock, 1991). Thus, it was hypothesized that the impact of chair-based leg-fidgeting could be different by age groups.
The objective of this study was to evaluate the effects of chair-based leg-fidgeting and age on frontal brain activities and cognitive function (executive function and working memory). It is hypothesized that exposure to chair-based fidgeting would affect frontal brain activities and improve executive function and working memory. Another hypothesis was that the effect of chair-based fidgeting would depend on the age of users.
Ⅱ. Methods
1. Participants
A total of 12 participants (6 young adults and 6 older adults) were recruited for this study. Table 1 shows the demographic and anthropometric information of participants. All participants did not have current pain (past seven days), history of musculoskeletal disorders, and history of drug or alcohol abuse. The study was approved by Institutional Review Board. All participants gave their written consent before the data collection. The consent form consisted of the participant’s right to continue or terminate the study, potential risks, benefits, and a description of the study methods were stated.
A repeated-measures experiment was conducted to determine the effects of chair-based fidgeting and age on frontal brain activities and cognitive performance. Each participant was randomly assigned to either a conventional chair or a chair with an under-the-table leg-fidgeting bar on separate days to minimize a systematic bias due to the order. All 12 participants experienced both conditions, with and without under-the-table leg-fidgeting bar. The sitting workstation, including the chair, table, and monitor, was set to fit each participant’s body according to the ANSI/HFES 100-2007 technical standards (ANSI/HFES 100, 2007). For the chair-based fidgeting condition, the floor-based HOVR (Active Ideas LLC, Chicago, IL) device was placed underneath the desk. The device included two separate paddles that encouraged the continuous movements of the feet. These paddles were positioned 5cm above the ground. The device enabled participants to do leg-swinging by engaging leg and thigh muscles. The height of the foot pedals was adjusted to fit the participant per the manufacturer’s recommendations. Participants had a practice session to be accustomed to the device and activities. During the main session (15 minutes) and the neurocognitive test session (25 minutes), participants were asked to constantly engage with the fidgeting activities while performing the tasks.
The participant had 15-minute of continuous exposure to the sitting condition to which they were assigned. Meanwhile, participants did typical office work such as the typing of standardized documents. Once they completed the 15-minute exposure of each chair condition, participants were asked to conduct the neurocognitive test protocol via the psychology experiment building language (PEBP) (Mueller & Piper, 2014). A neurocognitive test battery consisting of five different tasks was utilized to assess executive function and working memory (Kuppam et al., 2019; Penumudi et al., 2018). Participants were instructed to complete the test battery as quickly and accurately as possible. The duration of the neurocognitive test battery was approximately 25 minutes.
The functional Near Infrared Spectroscopy (fNIRS) (fNIR 400 system; Biopac Systems, Inc.; Santa Barbara, California) was utilized to measure the prefrontal cortex activity of the participants while performing the neurocognitive test battery. The fNIRS headband consisted of four infrared light sources and 10 detectors (16 active optodes), which were placed bilaterally on the participant’s forehead according to the international 10-20 EEG procedure of electrode placement (F7, Fp1, Fp2, F8 and Brodmann’s areas 9, 10, 45, 46) (Rodrigo et al., 2016). Based on the modified Beer-Lamber Law, the relative concentrations of average oxygenation values (µmol/L) on the prefrontal cortex were computed (Cope & Delpy, 1988). A motion artifact cancellation filter was applied to reduce the noise due to the movement. Baseline cerebral oxygenation levels were obtained before conducting each neurocognitive task to normalize the values (Izzetoglu et al., 2005; Kim et al., 2018). Participants stared at the white wall for 30 seconds during the baseline period. The normalized total hemoglobin (HbT), oxygenated hemoglobin (HbO2), reduced hemoglobin (HbR), and oxygenation changes (Oxy) were analyzed. These measures were grouped into four areas, including left lateral (optodes from 1 to 4), left central (optodes from 5 to 8), right central (optodes from 9 to 12), and right lateral hemisphere (optodes from 13 to 16) for the analysis of different prefrontal regions. For each region, the mean value of hemodynamic measures was calculated.
The neurocognitive test battery consisted of five tasks, including Wisconsin Cart Sort, Flanker, Memory Span, Trail-Making, and Stroop Color Word tasks. For the Wisconsin Cart Sort task (Huizinga et al., 2006), participants were asked to adequately sort the cards by applying three rules, including the number of shapes, the color of shapes, and the shape itself. This rule was hidden and changed throughout the tasks. Based on trial-and-error, participants recognized the rule and kept playing it. For the Flanker task (ŻUrawska Vel Grajewska et al., 2011), participants identified the direction of a central arrow. Five arrows were directed at the same direction (i.e., congruent) or at the opposite direction (i.e., incongruent) each time. For the Memory Span task (Dempster, 1981), the different image was displayed in a sequence, and the participants memorized the order of the images. Depending on the participants’ achievement, the number of images varied from 3 to 9. For the Trail-Making task (Shibuya-Tayoshi et al., 2007), the participants connected dots with three different rules, including the sequence of numbers, letters, and alternated numbers-letters. For the Stroop Color Word task (Huizinga et al., 2006), the participants identified the color of the stimulus each time. One of four different colors (red, green, blue, yellow) appeared with the color names, which were matched or unmatched with the stimulus color. For the cognitive performance measures, the mean reaction time (milliseconds), the proportion of correct responses (%), and the total completion time (seconds) were analyzed.
2. Analysis
The normality of measures was initially diagnosed. For the data with non-normality, the Johnson transformations were employed to meet the assumptions of the parametric analysis. analysis of variance (ANOVA) was used to evaluate the effect of the fidgeting condition and age on fNIR and cognitive performance measures. The fidgeting condition and age were determined as fixed effects, and the participants were set as a random effect. For non-normal data, the Kruskal-Wallis tests were conducted. The statistical significance (p-value) was set as .05.
Ⅲ. Results
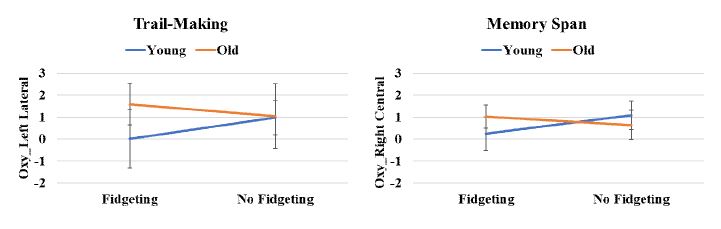
1. fNIR Data
For the Wisconsin Card Sort task, Oxy in the left central hemisphere was significantly different by the fidgeting condition (p=.031) (Figure 1). For the Flanker task, HbT in the left lateral prefrontal cortex (p=.03) and Oxy in the right lateral prefrontal cortex (p=.027) were significantly influenced by age. For the Memory Span task, Oxy in the right lateral prefrontal cortex (p=.046) was significantly affected by age, whereas Oxy in the right central prefrontal cortex (p=.021) was significantly different by the interaction effect of the fidgeting and age (Figure 2). For the Trail-Making task, Oxy in the left lateral prefrontal cortex (p=.046) was significantly affected by the interaction effects of the fidgeting and age (Figure 2). For the Stroop Color Word task, HbO2 in the left lateral (p=.036), Oxy in the left lateral (p=.013), and Oxy in the right lateral (p=0.012) prefrontal cortex were significantly affected by the fidgeting condition (Figure 1). The HbR in left central (p=.004) and right central (p=.006) prefrontal cortex significantly varied by the age.
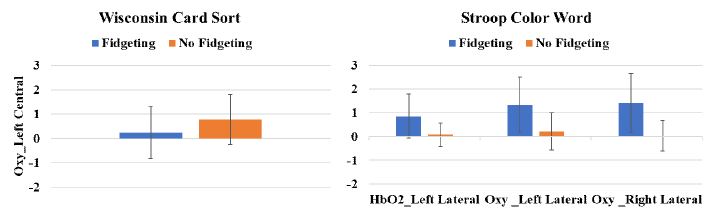
Main effects of fidgeting condition on Oxy at the left central prefrontal cortex in Wisconsin Card Sort task, and HbO2 at left lateral, Oxy at the left lateral and right lateral prefrontal cortex in Stroop Color Word taskNote. The mean and standard error were displayed.
2. Cognitive Performance
For the Wisconsin Card Sort task, the mean reaction time of correct (p=.015) and incorrect (p=.018) answers were significantly different by age (Table 2). Older adults showed greater reaction time of correct (Older: 1557.9±356.2ms vs. Young: 1067.1±267.2ms) and incorrect answers (Older: 1573.7±357.6ms vs. Young: 1082.8±274.7ms) than young adults. For the Flanker task, mean reaction time of congruent (p=.019) and incongruent (p=.044) conditions were significantly affected by age. Older adults showed greater reaction time of congruent (Older: 505.5±53.0ms vs. Young: 428.6±41.9ms) and incongruent conditions (Older: 562.8±56.7ms vs. Young: 493.5±45.9ms) than young adults. For the Memory Span, Trail-Making, and Stroop Color Word tasks, there were no significant differences of the cognitive performance measures by the fidgeting condition and age.
Ⅳ. Discussion
This study investigated the effect of chair-based fidgeting and age on hemodynamic responses on the prefrontal brain and cognitive performance of five neurocognitive tasks. The results showed that the prefrontal brain activities (HbO2 and Oxy measures) were significantly affected by the fidgeting condition. The effects of fidgeting differed by age groups, particularly during Trail-Making and Memory Span tasks, which required executive function and working memory.
For the Stroop Color Word task, there was a significant increase of HbO2 (oxygenated hemoglobin) at the left lateral prefrontal cortex and Oxy (oxygenation changes) at the left lateral and right lateral prefrontal cortex with the chair-based fidgeting compared to the no fidgeting condition. This was similar to the previous fMRI study showing that the brain activation in the left lateral prefrontal cortex was positively associated with age during the Stroop Color Word task (Adleman et al., 2002). The cognitive processes underlying the Stroop Color Word task included the executive function (e.g., response inhibition, inference resolution, behavioral conflict resolution), and the frontal lobe is known to mediate this cognitive process (Adleman et al., 2002). Especially, lateral prefrontal regions were known to be associated with inference processing, response inhibition, and word reading, which was aligned with our study findings (Pujol et al., 2001). In terms of cognitive performance, there were no significant differences by the fidgeting condition in the present study. This indicates that the chair-based fidgeting increased the prefrontal brain activation to recruit appropriate neural resources for the comparable task performance with the no fidgeting condition.
For the Memory Span task, an opposite pattern existed between young and older adults by a chair-based fidgeting condition. With chair-based fidgeting, the older adults showed the increased Oxy values in the right central prefrontal cortex, whereas the young adults showed the decreased Oxy values compared to no fidgeting condition. This was consistent with the previous fMRI study showing brain activity was lateralized to the right hemisphere during the high-load (e.g., six-letter memory) working memory task (Rypma & D’Esposito, 1999). There were no differences in cognitive performance measures by fidgeting condition and age groups during the Memory Span task. This suggests that older adults recruited more neural resources with the chair-based fidgeting to maintain a comparable cognitive performance (i.e., short-term working memory) as the no fidgeting condition. In contrast, young adults’ decreased prefrontal brain activation with the chair-based fidgeting may indicate the improved efficiency of the prefrontal cortex instead of mobilizing more neural resources (Zhao et al., 2020).
For the Trail-Making (i.e., cognitive set-shifting) task, the effects of fidgeting on the prefrontal brain activities depended on age group. Older adults revealed an increased Oxy at the left lateral prefrontal cortex with the fidgeting, whereas young adults showed a decreased Oxy with the fidgeting relative to no fidgeting condition. This was aligned with the previous fMRI study showing that brain activities of the lateral prefrontal cortex region were associated with the Trail-Making test (Yochim et al., 2007). There was no significant difference in cognitive performance by fidgeting condition and age group. This suggests that older adults’ lateral prefrontal cortex was more involved with fidgeting to complete the Trail-Making task. The fidgeting may be beneficial to prevent the set-shifting deficit by recruiting additional neural resources.
The cognitive performance of neurocognitive tests (executive functioning and working memory) was not affected by the fidgeting condition. Although there were age-related differences in mean reaction time during Wisconsin Card Sort and Flanker tasks, the fidgeting condition did not significantly alter the cognitive performance. This was similar to the previous study showing that cognitive performance of computer-based tasks was not reduced with the use of a chair-based fidgeting device (Kar et al., 2018). This indicates that the adoption of the chair-based fidgeting device would not negatively impact the cognitive performance and task performance of computed-based office tasks.
There were several limitations worth to be noted. First, the short-term effect of the fidgeting was only assessed in this study. While we carefully controlled the environment in the laboratory condition and assessed the short-term impact, the longer-term impact of the fidgeting devices on brain function is not known yet. Second, healthy young and older adults were only recruited in this study. Given the results of this study, fidgeting intervention may be beneficial to patients with cognitive deficits by encouraging them to recruit more appropriate neural resources during the cognitive tasks.
In conclusion, chair-based fidgeting increased the brain activation in the lateral prefrontal cortex while performing executive functioning-oriented tasks. The impacts of fidgeting on the prefrontal brain activities depended on the age group. With fidgeting, older adults showed increased prefrontal brain activities during cognitive tasks involving executive functioning and working memory compared to no fidgeting condition. There was no negative impact of fidgeting on cognitive performance. Fidgeting intervention may be beneficial to encourage older adults to recruit more neural resources and prevent the potential cognitive deficit. Changes in brain cortical activities with fidgeting in vulnerable populations (e.g., dementia, Parkinson’s disease etc.) have not been well studied. Therefore, this research may provide a starting point to develop better cognitive treatment approaches to support people with speech or language related cognitive deficits. Furthermore, future work including larger cohorts and neurogenic populations is necessary to explore more valid motor-cognitive enhanced approaches.
Acknowledgments
This work was supported by research and Artistry Grant in Northern Illinois University.
References
-
Adleman, N. E., Menon, V., Blasey, C. M., White, C. D., Warsofsky, I. S., Glover, G. H., & Reiss, A. L. (2002). A developmental fMRI study of the stroop Color-Word task. NeuroImage, 16(1), 61–75.
[https://doi.org/10.1006/nimg.2001.1046]

-
Andersen, J. H., Fallentin, N., Thomsen, J. F., & Mikkelsen, S. (2011). Risk factors for neck and upper extremity disorders among computer users and the effect of interventions: An overview of systematic reviews. Occupational and Environmental Medicine, 68(Suppl_1), A115.
[https://doi.org/10.1136/oemed-2011-100382.383]

-
Best, J. R. (2010). Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Developmental Review, 30(4), 331–351.
[https://doi.org/10.1016/j.dr.2010.08.001]

-
Brown, W. J., Miller, Y. D., & Miller, R. (2003). Sitting time and work patterns as indicators of overweight and obesity in Australian adults. International Journal of Obesity, 27(11), 1340–1346.
[https://doi.org/10.1038/sj.ijo.0802426]

-
Bryan, J., & Luszcz, M. A. (2000). Measurement of executive function: considerations for detecting adult age differences. Journal of Clinical and Experimental Neuropsychology, 22(1), 40-55.
[https://doi.org/10.1076/1380-3395(200002)22:1;1-8;FT040]

-
Buckley, J. P., Mellor, D. D., Morris, M., & Joseph, F. (2013). Standing-based office work shows encouraging signs of attenuating post-prandial glycaemic excursion. Occupational and Environmental Medicine, 71(2), 109–111.
[https://doi.org/10.1136/oemed-2013-101823]

-
Carpenter, P. (2000). Working memory and executive function: Evidence from neuroimaging. Current Opinion in Neurobiology, 10(2), 195–199.
[https://doi.org/10.1016/S0959-4388(00)00074-X]

-
Cope, M., & Delpy, D. T. (1988). System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Medical & Biological Engineering & Computing, 26(3), 289–294.
[https://doi.org/10.1007/BF02447083]

-
Davis, C. L., Tomporowski, P. D., McDowell, J. E., Austin, B. P., Miller, P. H., Yanasak, N. E., . . . & Naglieri, J. A. (2011). Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychology, 30(1), 91–98.
[https://doi.org/10.1037/a0021766]

-
Dempster, F. N. (1981). Memory span: Sources of individual and developmental differences. Psychological Bulletin, 89(1), 63–100.
[https://doi.org/10.1037/0033-2909.89.1.63]

-
D’Esposito, M., Detre, J. A., Alsop, D. C., Shin, R. K., Atlas, S., & Grossman, M. (1995). The neural basis of the central executive system of working memory. Nature, 378(6554), 279–281.
[https://doi.org/10.1038/378279a0]

-
Dunstan, D. W., Kingwell, B. A., Larsen, R., Healy, G. N., Cerin, E., Hamilton, M. T., . . . Owen, N. (2012). Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care, 35(5), 976–983.
[https://doi.org/10.2337/dc11-1931]

-
Falck, R. S., Davis, J. C., & Liu-Ambrose, T. (2016). What is the association between sedentary behaviour and cognitive function? A systematic review. British Journal of Sports Medicine, 51(10), 800–811.
[https://doi.org/10.1136/bjsports-2015-095551]

-
Hamilton, M. T., Hamilton, D. G., & Zderic, T. W. (2007). Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes, 56(11), 2655–2667.
[https://doi.org/10.2337/db07-0882]

-
Huizinga, M., Dolan, C. V., & van der Molen, M. W. (2006). Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia, 44(11), 2017–2036.
[https://doi.org/10.1016/j.neuropsychologia.2006.01.010]

-
Izzetoglu, M., Devaraj, A., Bunce, S., & Onaral, B. (2005). Motion artifact cancellation in NIR spectroscopy using wiener filtering. IEEE Transactions on Biomedical Engineering, 52(5), 934–938.
[https://doi.org/10.1109/TBME.2005.845243]

-
Júdice, P. B., Hamilton, M. T., Sardinha, L. B., & Silva, A. M. (2015). Randomized controlled pilot of an intervention to reduce and break-up overweight/obese adults’ overall sitting-time. Trials, 16(1).
[https://doi.org/10.1186/s13063-015-1015-4]

-
Kar, G., Sun, Y., Celikors, E., Villacreces, P., Misailedes, E., Schatz, M., K., . . . & Hedge, A. (2018). Effects of a dynamic foot movement device on cognitive performance in short-duration computer-based tasks. Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 62(1), 369–372.
[https://doi.org/10.1177/1541931218621085]

-
Kim, I. S., Millin, N. J., & Hwang, J. (2018). Word retrieval by verbal fluency tasks for young and old people: An fNIR study. Clinical Archives of Communication Disorders, 3(1), 52–58.
[https://doi.org/10.21849/cacd.2018.00318]

-
Koepp, G. A., Moore, G. K., & Levine, J. A. (2016). Chair-based fidgeting and energy expenditure. BMJ Open Sport & Exercise Medicine, 2(1), e000152.
[https://doi.org/10.1136/bmjsem-2016-000152]

-
Kuppam, V. A., Kim, I. S., Penumudi, S. A., & Hwang, J. (2019). Effects of leaning workstation on oxygenation in the prefrontal cortex and cognitive performance. Clinical Archives of Communication Disorders, 4(2), 83–89.
[https://doi.org/10.21849/cacd.2019.00073]

-
Le, P., & Marras, W. S. (2016). Evaluating the low back biomechanics of three different office workstations: Seated, standing, and perching. Applied Ergonomics, 56, 170–178.
[https://doi.org/10.1016/j.apergo.2016.04.001]

-
Levine, J. A., Schleusner, S. J., & Jensen, M. D. (2000). Energy expenditure of nonexercise activity. The American Journal of Clinical Nutrition, 72(6), 1451–1454.
[https://doi.org/10.1093/ajcn/72.6.1451]

-
McMorris, T., Sproule, J., Turner, A., & Hale, B. J. (2011). Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiology & Behavior, 102(3–4), 421–428.
[https://doi.org/10.1016/j.physbeh.2010.12.007]

-
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100.
[https://doi.org/10.1006/cogp.1999.0734]

-
Mörl, F., & Bradl, I. (2013). Lumbar posture and muscular activity while sitting during office work. Journal of Electromyography and Kinesiology, 23(2), 362–368.
[https://doi.org/10.1016/j.jelekin.2012.10.002]

-
Mueller, S. T., & Piper, B. J. (2014). The psychology experiment building language (PEBL) and PEBL test battery. Journal of Neuroscience Methods, 222, 250–259.
[https://doi.org/10.1016/j.jneumeth.2013.10.024]

-
Noma, H., Ohmura, A., Kuwahara, N., & Kogure, K. (2004). Wearable sensors for auto-event-recording on medical nursing: User study of ergonomic design. Eighth International Symposium on Wearable Computers.
[https://doi.org/10.1109/ISWC.2004.48]

-
Penumudi, S. A., Kim, I. S., Chodraju, S. S. R., Mohler, R., & Hwang, J. (2018). Parkinson’s patients’ cognitive and motor function while using a mouse and touchscreen. Clinical Archives of Communication Disorders, 3(2), 151–157.
[https://doi.org/10.21849/cacd.2018.00388]

-
Pujol, J., Vendrell, P., Deus, J., Junqué, C., Bello, J., Martı́-Vilalta, J. L., & Capdevila, A. (2001). The effect of medial frontal and posterior parietal demyelinating lesions on stroop interference. NeuroImage, 13(1), 68–75.
[https://doi.org/10.1006/nimg.2000.0662]

-
Pynt, J. (2015). Rethinking design parameters in the search for optimal dynamic seating. Journal of Bodywork and Movement Therapies, 19(2), 291–303.
[https://doi.org/10.1016/j.jbmt.2014.07.001]

-
Rodrigo, A. H., Ayaz, H., & Ruocco, A. C. (2016). Examining the neural correlates of incidental facial emotion encoding within the prefrontal cortex using functional Near-Infrared Spectroscopy. Lecture Notes in Computer Science, 102–112.
[https://doi.org/10.1007/978-3-319-39955-3_10]

-
Rypma, B., & D’Esposito, M. (1999). The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proceedings of the National Academy of Sciences, 96(11), 6558–6563.
[https://doi.org/10.1073/pnas]

-
Salthouse, T. A., & Babcock, R. L. (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27(5), 763.
[https://doi.org/10.1037/0012-1649.27.5.763]

-
Saraiva, M., Vilas-Boas, J. P., & Castro, M. A. (2021). Prefrontal cortex oxygenation and sleep quality in cognitive dual-task—fNIR study. European Journal of Public Health, 31(Supplement_2).
[https://doi.org/10.1093/eurpub/ckab120.031]

-
Shibuya-Tayoshi, S., Sumitani, S., Kikuchi, K., Tanaka, T., Tayoshi, S., Ueno, S. I., & Ohmori, T. (2007). Activation of the prefrontal cortex during the Trail-Making test detected with multichannel near-infrared spectroscopy. Psychiatry and Clinical Neurosciences, 61(6), 616–621.
[https://doi.org/10.1111/j.1440-1819.2007.01727.x]

- ANSI/HFES 100. (2007). ANSI/HFES 100–2007: Human factors engineering of computer workstations. Human Factors and Ergonomics Society.
-
Yochim, B., Baldo, J., Nelson, A., & Delis, D. C. (2007). D-KEFS trail making test performance in patients with lateral prefrontal cortex lesions. Journal of the International Neuropsychological Society, 13(4), 704-709.
[https://doi.org/10.1017/S1355617707070907]

-
Zhao, W., Zhang, Q., Chen, X., Li, Y., Li, X., Du, B., . . . & Li, J. (2020). The VNTR of the AS3MT gene is associated with brain activations during a memory span task and their training-induced plasticity. Psychological Medicine, 51(11), 1927–1932.
[https://doi.org/10.1017/S0033291720000720]

-
ŻUrawska Vel Grajewska, B., Sim, E. J., Hoenig, K., Herrnberger, B., & Kiefer, M. (2011). Mechanisms underlying flexible adaptation of cognitive control: Behavioral and neuroimaging evidence in a flanker task. Brain Research, 1421, 52–65.
[https://doi.org/10.1016/j.brainres.2011.09.022]