Unilateral Middle Cerebral Artery Infarction and Airway Protection in Swallowing
Copyright 2025 ⓒ Korean Speech-Language & Hearing Association.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The relationship between the stroke site of the lesion and swallowing pathophysiology was not well understood. The swallowing pathophysiology such as airway protection, may be related to the hemispheric lesion. To understand the hemispheric effects on airway protection after middle cerebral artery (MCA) infarction, the purpose of this study was to examine whether there is a difference in two temporal measurements of laryngeal vestibule closure: initiation of laryngeal closure (ILC) and laryngeal closure duration (LCD) between left and right MCA hemispheric stroke.
This study examined the ILC and LCD between 30 stroke survivors with left or right MCA hemispheric lesions. Means and standard deviations of ILC and LCD were analyzed on 2 ml and 5 ml thin liquid boluses. Statistical comparisons were used by repeated analysis of variance. A significant level was set at p<.05.
Stroke survivors with left MCA lesions displayed significantly delayed initiation of laryngeal closure than those with right MCA lesions. There was a volume difference in ILC. However, there were no differences in LCD for both the location of the lesion and bolus volume.
The differences observed in hemispheric involvement in ILC indicate that stroke survivors with left MCA hemispheric lesions have a slower swallow response which puts them at higher risk of aspiration. Reduced oral movement and sensation in stroke survivors with left MCA hemispheric lesions may influence delayed initiation of laryngeal closure.
Keywords:
Dysphagia, laryngeal closure, airway protection, stroke, middle cerebral arteryⅠ. Introduction
The cortical controls of the swallowing process are complex. The cortical lesion is likely to disrupt the physiological and biomechanical complexity of swallowing (Jean, 2001). Researchers have investigated the cortical control in swallowing in terms of different stroke lesions. Specifically, cortical laterality has been investigated due to one of the organizational principles of the cortex (Galaburda et al., 1990). Laterality is the relationship between controlling the activity and dominance of one side of the cortex. Previous research focused on the relationship between swallowing and hemispheric dominance using analysis of cortical activation through imaging technologies. The results of the studies in the normal population varied. Some reported that the left hemisphere dominantly controls the oral stage, and the right hemisphere influences the pharyngeal stage (Kern et al., 2001; May et al., 2017; Teismann et al., 2011; Wilmskoetter et al., 2018). Others stated that the swallowing process is not responsible for the dominant hemisphere, as various areas at both hemispheres are activated during the oropharyngeal swallowing (Hamdy et al., 1996; Martin et al., 2001).
The hemispheric dominance in the specific swallowing impairment presented in stroke survivors. However, the findings are inconsistent with the imaging analysis (Chen et al., 1990; Daniels et al., 1996, 2017; Han et al., 2018; Jeon et al., 2014). Some studies used the Modified Barium Swallow Impairment Profile (MBSImP) and temporal measurements to compare the swallowing physiologic differences between the right and left hemisphere lesions. Daniels and her colleagues reported that the pharyngeal impairment score of MBSImP did not differ in the lesion location (Daniels et al., 2017). Temporal analysis by Han and her colleagues reported that the bolus transition in the oropharynx was not significantly different between right and left hemisphere lesions (Han et al., 2018). On the other hand, some studies reported that the patients with the right hemisphere lesions had more pharyngeal impairment such as delayed pharyngeal swallowing, pharyngeal residue, and decreased hyolaryngeal impairments (Chen et al., 1990; Daniels et al., 1996; Jeon et al., 2014; Robbins et al., 1993; Wilmskoetter et al., 2018). The inconsistency might be related to research methods and heterogeneous lesions in subject populations. Investigating the relationship between homogeneous groups and specifically targeted swallowing physiology is important.
Acute stroke commonly involves the middle cerebral arteries (MCA) (Nogles & Galuska, 2023). MCA supplies the sensory-motor cortex, Broca’s, and Wernicke’s areas. These areas are critical because they are responsible for cognition, motor, speech, and language expression and comprehension (Moore & Dalley, 1999). Consequently, stroke survivors with MCA lesions often showed communication difficulties. Among them, the left MCA lesion predominantly results in different types of aphasia (Kelly et al., 2011; Kim et al., 2019; Somasundaram et al., 2014). Poor communication ability in patients with MCA stroke challenges the clinician in swallowing evaluation and rehabilitation (Somasundaram et al., 2014; Wilmskoetter et al., 2018). Considering the clinical challenges in MCA lesions and the limitations of previous studies regarding the lesions, it is necessary to investigate sufficient studies to target the relationship between the MCA lesion and swallowing pathophysiology.
Among acute stroke survivors with dysphagia, up to 70% of stroke survivors with dysphagia showed aspiration of food or liquid and some resulted in aspiration pneumonia, dehydration, malnutrition, and even death (Osawa et al., 2013). Stroke survivors with dysphagia suffer from poor or failed airway protection during swallowing (Kendall & Leonard, 2001; Park et al., 2010). It leads them to aspiration or penetration during swallowing. Airway protection is a critical physiological event as the clinician decides on the oral intake of food. The interruptions of airway protection are decisive factors in the quality of life of stroke survivors.
This investigation was mainly interested in two temporal measurements, the initiation and duration of laryngeal closure to examine the relationship between swallowing pathophysiology and MCA hemispheric stroke. Temporal measurements of laryngeal closure can help the precise diagnosis and guide the management of airway protection. Initiation of laryngeal closure (ILC) is the timing of the onset of laryngeal closure and laryngeal closure duration (LCD) is the duration of contacting the arytenoid cartilages to the epiglottis until reopening the larynx (Park et al., 2017). In addition, the bolus volume effect was examined. The null hypothesis was that there were no differences in ILC and LCD between left and right MCA hemispheric lesions of stroke survivors.
Ⅱ. Methods
1. Subjects
Thirty stroke survivors with middle cerebral artery (MCA) hemispheric infarction were selected for this investigation from the following inclusion criteria: 1) no history of previous stroke or other diseases that could affect swallowing function, 2) a completed swallowing examination at acute or subacute stages of stroke, and 3) confirmed unilateral cortical lesion using Computerized Tomography (CT) scans and / or Magnetic Resonance Imaging (MRI). Stroke survivors in this sample were divided into two groups: 14 left MCA hemispheric stroke survivors and 16 right MCA hemispheric stroke survivors. The mean age for left MCA hemispheric stroke was 68 years and the mean age for right MCA hemispheric stroke was 73 years. Table 1 gives the subject information.
2. Videofluoroscopic swallowing examination (VFSE)
Videofluoroscopic swallowing examinations were performed at Seoul National University Medical Center, Seoul, South Korea. Each subject was seated upright in a wheelchair or stretcher chair for the examination. The fluoroscopic tube was focused in the lateral plane on the oral cavity (the lips anteriorly to the pharyngeal wall posteriorly) and the nasopharynx (superiorly) to below the upper esophageal sphincter area (inferiorly). Boluses were presented in 2 ml and 5 ml thin liquid for two trials for each volume. The thin liquid was a mixture of water and barium sulfate powder (35% w/v). Each subject swallowed the bolus after placing the liquid in his / her mouth after the clinician delivered it with a spoon. Two trials for each volume were performed. A total of 120 swallows were submitted for analysis for this investigation. Frame-by-frame images were acquired for digital imaging files using a computer-based image processing system equipped with a digital computer frame grabber board (Pegasus HD / SD Board, Grass Valley Inc., Honorine, France) and image processing software (EDIUS 4.5, Grass Valley Inc.). The X-ray voltage was set at a 40-kV peak, which allowed the soft tissue of the pharynx to be visualized. Videofluoroscopic swallow examination was recorded at 30 frames per second.
3. Measurements
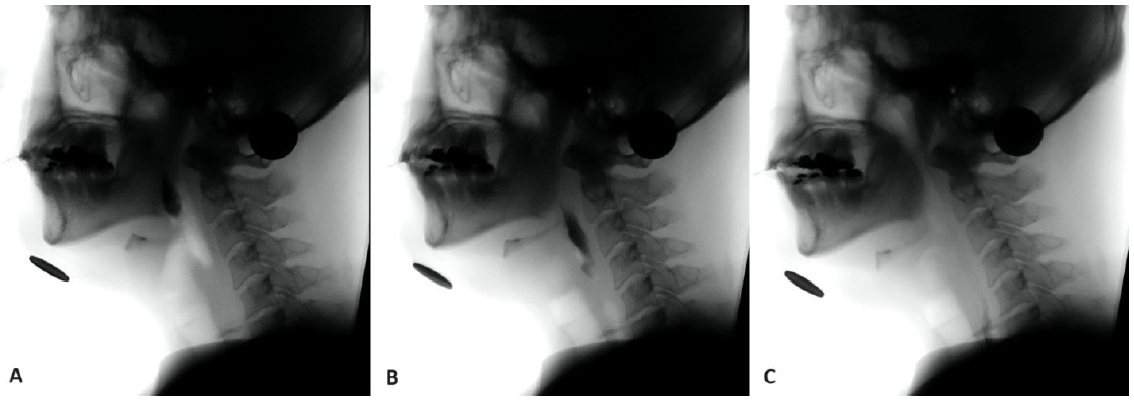
This investigation was focused on two measurements of laryngeal closure: initiation of laryngeal closure (ILC) and laryngeal closure duration (LCD). ILC was the time between initial contact of arytenoids and epiglottis and bolus head passing the ramus of the mandible (Park et al., 2017). LCD was the time between the last contact and the initial contact of the arytenoids and epiglottis (Kendall et al., 2004; Perlman et al., 1994). To accurately analyze the temporal sequence of events, slow motion, and frame-by-frame analyses were performed using a 100-ms video timer. Adobe Premier Pro CS5 (San Jose, CA) was used. First, each swallow was analyzed for the following three points of occurrence: A) bolus passing the ramus of the mandible, B) the first contact of the arytenoids and epiglottis, and C) the last moment of contact between the arytenoids and epiglottis. Second, the times for each of the abovementioned markers were recorded and computed using these formulas: ILC=(B)–(A), and LCD=(C)–(B).
The occurrence of penetration and aspiration were analyzed. Aspiration was defined as the entry of the bolus below the true vocal folds. Penetration was defined as the entry of the bolus into the vestibule, but not below the true vocal folds (Rosenbek, Robbins et al., 1996).
4. Statistical analysis
Statistical comparisons were completed using repeated measures analysis of variance (ANOVA) to determine group and volume effects. The significance level was set at p<.05. All the swallows for each subject on each bolus volume were analyzed separately.
Ⅲ. Results
1. Reliability and Normality
For intra-judge reliability, the investigator randomly selected and reanalyzed 20% of the subjects. The primary investigator repeated to analyze the selected subject. The value of Cronbach’s Alpha for the ILC was .996 and for LCD was .983. For inter-judge reliability, a second judge analyzed the same subjects. The judge’s results were compared with the results of the primary investigator. The value of Cronbach’s Alpha for ILC was .984 and for LCD was .991. Kolmogorov-Smirnov was performed and showed that the distribution of ILC and LCD departed significantly from normality (p<.001).
2. Initiation of laryngeal closure
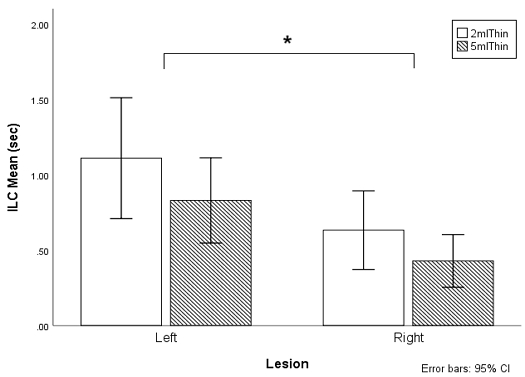
The stroke survivors with left MCA lesions demonstrated significantly longer delays in ILC than those with right MCA lesions (F(1,43)=6.653, p=.013, partial eta-squared=.004). The mean ILC of the left MCA stroke survivors was .97 seconds (SD=.75), and the mean ILC of the right MCA stroke survivors was .53 seconds (SD=.52). The ILC was significantly longer as volume decreased from 5 ml to 2 ml (F(1,43)=7.103, p=.011). The mean ILC of the 2 ml thin liquid was .87 seconds (SD=1.21) and the mean ILC of the 5 ml thin liquid was .63 seconds (SD=.47). In addition, there was an interaction between the site the of lesion and bolus volume (F(1,43)=.177, p<.001) (Figure 2).
3. Laryngeal closure duration
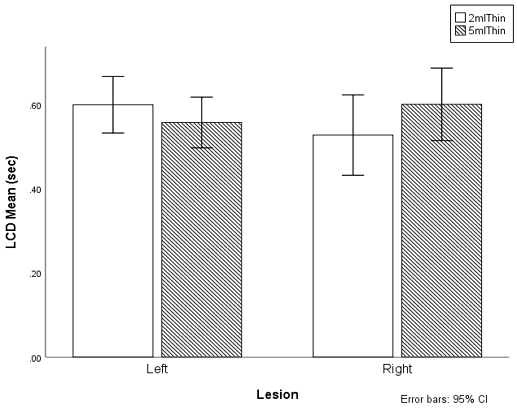
The laryngeal closure duration did not differ between left and right MCA lesions (F(1,47)=.090, p=.766) as well as between 2 ml and 5 ml volumes and lesion (F(1,47)=.412, p=.52, partial eta-squared=.107). The mean LCD of the left MCA stroke survivors was .58 seconds (SD=.15), and the mean LCD of the right MCA stroke survivors was .57 seconds (SD=.22). The mean LCD of the 2 ml thin liquid was .57 seconds (SD=.20) and the mean LCD of the 5 ml thin liquid was .58 seconds (SD=.22). In addition, there was an interaction between the site of the lesion and volume (F(1,47)=5.640, p<.001) (Figure 3). For the 2 ml thin, the left MCA lesion had longer LCD than those with the right MCA lesion. However, the right MCA lesion had longer LCD in 5 ml than those with the left MCA lesion.
4. Occurrence of penetration and aspiration
Eight stroke survivors with left MCA hemispheric lesions showed penetration and 6 stroke survivors with left MCA hemispheric lesions showed no penetration or aspiration. Eight stroke survivors with right MCA hemispheric lesions showed penetration and 3 stroke survivors with right MCA hemispheric lesions aspirated. Table 2 gives the number of subjects on occurrences of penetration and aspiration.
Ⅳ. Discussion and Conclusion
Stroke survivors with left MCA lesions had significantly delayed initiation of laryngeal closure than those with right MCA hemispheric lesions. This finding indicates that the left MCA hemisphere damage may be related to the slow response of laryngeal closure as bolus enters the pharynx during swallowing. In addition, there were volume effects. Initiation of laryngeal closure in 2 ml was significantly longer than in 5 ml thin liquid. This finding revealed that small volume requires a high threshold of neuromuscular activation in oropharyngeal structures to initiate the laryngeal closure effectively.
Delayed initiation of laryngeal closure in stroke survivors with a left MCA hemispheric lesion may indicate that stroke survivors with a left MCA hemispheric lesion have more difficulties in the process of oral sensory input to trigger the onset of laryngeal closure than those with a right MCA hemispheric lesion. Oral sensorimotor structures, such as the anterior faucial pillar, are critical for triggering the pharyngeal stage of swallowing (Miller, 2008). Sensory input from oral receptors is delivered to the cortex and brainstem, then the physiological events of the pharyngeal stage of swallowing are executed and organized through a central pattern generator (Barlow, 2009; Bautista et al., 2014; Wilmskoetter et al., 2020). When stroke survivors with left MCA hemispheric lesions do not receive enough sensory input from oral structures, the airway closure mechanism between epiglottis and arytenoid cartilages may be slowed or disorganized. Although oral sensation during the oral stage is functional in certain conditions or thresholds, poor tongue muscle strength and incoordination of oropharyngeal musculature may reflect a hesitation to initiate the pharyngeal stage. Consequently, the hyolaryngeal excursion is delayed and the epiglottis closes slowly. Additionally, MCA is connected to not only the lateral parts of the cortex but also the basal ganglia (Faiz et al., 2011; Snell, 2010). Somatosensory cortical areas of the left side that are involved with the MCA might predominate impact initiation and coordination of laryngeal closure.
The findings in this study are consistent with the previous investigations of hemispheric involvement in swallowing physiology. Left hemisphere stroke survivors had more oral abnormalities than right hemispheric stroke survivors. Robbins and Levine(1988) reported that stroke survivors with left hemisphere lesions showed more oral residue at the palate, velum, and under or on top of the tongue than those with right hemisphere lesions. In addition, stroke survivors with left hemispheric lesions struggled with initiating coordinating motor activity and showed apraxia (Robbins & Levine, 1988). Interestingly, in this investigation, 79% of stroke survivors with left hemisphere lesions had dysarthria, whereas 56% of stroke survivors with right hemisphere lesions did. The left MCA hemispheric lesion may impact more in weak oral strength and movement which is associated with delayed initiating of the laryngeal closure. On the other hand, stroke survivors with the right MCA hemispheric lesions showed relatively shorter initiation of laryngeal closure.
There was no difference in laryngeal closure duration between stroke survivors with left and right MCA hemispheric lesions in this study. Swallowing requires timely, coordinated, and sequential acts of the oropharyngeal structures. The brain responds to each swallowing stage from oral preparatory to esophageal stages. Each hemisphere has a different role in the swallowing stages (Dziewas et al., 2003; Hamdy et al., 1996). The results in this study supported that the left hemisphere region, including the MCA, may play an important role in the early stage of swallowing. Teismann et al.(2009) examined cortical brain activation while the normal subject swallowed the thin liquid. Only the left hemisphere was activated at the first 600ms, and then both hemispheres activated for 200ms. Left hemispheric activation occurs during the oral stage of swallowing and right hemispheric activation begins to involve in the pharyngeal stage of swallowing. In addition, Furlong et al.(2004) reported that activation shifted from left to right during the swallowing (Furlong et al., 2004). The intact hemispheric side of the brain may compensate for the damaged side of the brain during the laryngeal closure and/or bilateral activation helps the laryngeal closure duration to complete regardless of the side of the lesion.
The small volume took more time to initiate the laryngeal closure than the large volume. In this investigation, initiation of laryngeal closure of 2ml thin liquid was delayed 0.24 seconds more than that of 5ml thin liquid. The findings of this investigation confirmed that small volume requires a higher threshold to initiate the laryngeal closure than large volume (Ruak et al., 2003). Initiation of laryngeal closure occurs through the triggered hyolaryngeal excursion as the bolus touches the sensory receptors in the oral cavity, especially the anterior faucial pillar. The large volume is relatively able to touch the wide area in the oral cavity, which means more sensory receptors in the oral cavity are stimulated. This may help alert the bolus transition into the pharynx as well as quickly trigger laryngeal closure. In addition, large volumes may generate higher pressure than small volumes (Chen & Engelen, 2012). The high bolus pressure may contribute to quickly reaching the threshold of the triggering point of the initiation of laryngeal closure. This investigation includes two different volumes of thin liquid. Both volumes, 2ml and 5ml were relatively small compared to the regular sip of the liquid via cup. Further research needs to evaluate the initiation of laryngeal closure in various volumes and the consistency of bolus.
Even though stroke survivors with left MCA hemispheric lesions did show delayed initiation of laryngeal closure, none of these survivors showed aspiration. Three stroke survivors with right MCA hemispheric lesions aspirated the bolus silently. Among them, subjects number 18 and 25 showed short ILC which was a similar value to the normal population (0.19 seconds) or stroke survivors with non-aspirators (0.99 seconds) (Park et al., 2010). However, they showed residues in the base of the tongue, vallecula, and pharyngeal wall. The residue in the pharynx may result in aspiration after the swallow. Although their ILCs were close to the normal population and/or stroke survivors without aspiration, it is possible to have difficulties in the pharyngeal stage of swallowing. The short ILC may represent timely laryngeal closure to protect the airway for safe swallowing, however, this may not guarantee safe swallowing for the entire process of swallowing in stroke survivors. The clinicians should integrate other observations and measurements to understand the overall process of swallowing for appropriate swallowing diagnosis. It is necessary to study the relationship between swallowing pathophysiology and the occurrence of aspiration. ILC is shown to be related to aspiration before the swallow.
The result of this study may help develop plans for dysphagia diagnosis and treatment. For diagnosis, the clinician should carefully evaluate oral sensory and motor function in stroke survivors with left MCA hemispheric lesions. Delayed sensorimotor function of the anterior faucial pillar, which is the swallowing trigger point in the oral cavity, may slow the swallowing response of laryngeal closure in stroke survivors with left MCA hemispheric lesions. Dysphagia management for stroke survivors with left MCA hemispheric lesions would include taste and tactile stimulation, which can enhance their declined oral sensitivity to help the efficient laryngeal closure (Rosenbek, Roecker et al., 1996). For example, using the ice chip is one of the strategies to stimulate the swallowing responses (Pisegna & Langmore, 2018). In addition, compensatory strategies such as chin down will help the pharyngeal swallow effectively by widening the vallecula (Macrae et al., 2014).
This investigation has several limitations. This investigation did not control the extent of the stroke lesion and the days between the onset of the stroke and the swallowing evaluation date. In addition, a small sample size was analyzed. It is necessary to investigate timing differences in swallowing with larger and controlled samples. Future research would focus on the impact of MCA lesions on other aspects of swallowing physiology and pathophysiology. In addition, the bolus consistency was limited to thin liquids in this study, and it is necessary to examine various bolus consistencies and volumes.
Acknowledgments
This study did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
-
Barlow, S. M. (2009). Central pattern generation involved in oral and respiratory control for feeding in the term infant. Current Opinion in Otolaryngology & Head and Neck Surgery, 17(3), 187-193.
[https://doi.org/10.1097/MOO.0b013e32832b312a]

-
Bautista, T. G., Sun, Q.-J., & Pilowsky, P. M. (2014). The generation of pharyngeal phase of swallow and its coordination with breathing: Interaction between the swallow and respiratory central pattern generators. Progress in Brain Research, 212, 253-275.
[https://doi.org/10.1016/B978-0-444-63488-7.00013-6]

-
Chen, J., & Engelen, L. (Eds.). (2012). Food oral processing: Fundamentals of eating and sensory perception. Chichester, UK: Wiley-Blackwell.
[https://doi.org/10.1002/9781444360943]

-
Chen, M. Y., Ott, D. J., Peele, V. N., & Gelfand, D. W. (1990). Oropharynx in patients with cerebrovascular disease: Evaluation with videofluoroscopy. Radiology, 176(3), 641-643.
[https://doi.org/10.1148/radiology.176.3.2389021]

-
Daniels, S. K., Foundas, A. L., Iglesia, G. C., & Sullivan, M. A. (1996). Lesion site in unilateral stroke patients with dysphagia. Journal of Stroke and Cerebrovascular Diseases, 6(1), 30-34.
[https://doi.org/10.1016/S10523057(96)80023-1]

-
Daniels, S. K., Pathak, S., Mukhi, S. V., Stach, C. B., Morgan, R. O., & Anderson, J. A. (2017). The relationship between lesion localization and dysphagia in acute stroke. Dysphagia, 32(6), 777-784.
[https://doi.org/10.1007/s00455-017-9824-0]

-
Dziewas, R., Soros, P., Ishii, R., Chau, W., Henningsen, H., Ringelstein, E. B., . . . Pantev, C. (2003). Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage, 20(1), 135-144.
[https://doi.org/10.1016/S1053-8119(03)00285-4]

- Faiz, O., Blackburn, S., & Moffat, D. (2011). Anatomy at a glance (3rd ed.). Chichester, UK: Wiley Blackwell.
-
Furlong, P. L., Hobson, A. R., Aziz, Q., Barnes, G. R., Singh, K. D., Hillebrand, A., . . . Hamdy, S. (2004). Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. NeuroImage, 22(4), 1447-1455.
[https://doi.org/10.1016/j.neuroimage.2004.02.041]

-
Galaburda, A. M., Rosen, G. D., & Sherman, G. F. (1990). Individual variability in cortical organization: Its relationship to brain laterality and implications to function. Neuropsychologia, 28(6), 529-546.
[https://doi.org/10.1016/0028-3932(90)90032-J]

-
Hamdy, S., Aziz, Q., Rothwell, J. C., Singh, K. D., Barlow, J., Hughes, D. G., . . . Thompson, D. G. (1996). The cortical topography of human swallowing musculature in health and disease. Nature Medicine, 2(11), 1217-1224.
[https://doi.org/10.1038/nm1196-1217]

-
Han, H., Park, T., Oh, B.-M., Seo, H. G., & Kim, Y. (2018). Bolus transition during oropharyngeal swallowing after unilateral cortical stroke. Clinical Archives of Communication Disorders, 3(3), 178-184.
[https://doi.org/10.21849/cacd.2018.00423]

-
Jean, A. (2001). Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiological Reviews, 81(2), 929-969.
[https://doi.org/10.1152/physrev.2001.81.2.929]

-
Jeon, W. H., Park, G. W., Lee, J. H., Jeong, H. J., & Sim, Y. J. (2014). Association between location of brain lesion and clinical factors and findings of videofluoroscopic swallowing study in subacute stroke patients. Brain & NeuroRehabilitation, 7(1), 54-60.
[https://doi.org/10.12786/bn.2014.7.1.54]

-
Kelly, J., Wright, D., & Wood, J. (2011). Medicine administration errors in patients with dysphagia in secondary care: A multi-centre observational study. Journal of Advanced Nursing, 67(12), 2615-2627.
[https://doi.org/10.1111/j.1365-2648.2011.05700.x]

-
Kendall, K. A., & Leonard, R. J. (2001). Bolus transit and airway protection coordination in older dysphagic patients. The Laryngoscope, 111(11), 2017-2021.
[https://doi.org/10.1097/00005537-200111000-00028]

-
Kendall, K. A., Leonard, R. J., & McKenzie, S. (2004). Airway protection: Evaluation with videofluoroscopy. Dysphagia, 19(2), 65-70.
[https://doi.org/10.1007/s00455-003-0500-1]

-
Kern, M. K., Jaradeh, S., Arndorfer, R. C., & Shaker, R. (2001). Cerebral cortical representation of reflexive and volitional swallowing in humans. American Journal of Physiology-Gastrointestinal and Liver Physiology, 280(3), G354-G360.
[https://doi.org/10.1152/ajpgi.2001.280.3.G354]

-
Kim, J. H., Oh, S. H., Jeong, H. J., Sim, Y. J., Kim, D. G., & Kim, G. C. (2019). Association between duration of dysphagia recovery and lesion location on magnetic resonance imaging in patients with middle cerebral artery infarction. Annals of Rehabilitation Medicine, 43(2), 142-148.
[https://doi.org/10.5535/arm.2019.43.2.142]

-
Macrae, P., Anderson, C., & Humbert, I. (2014). Mechanisms of airway protection during chin-down swallowing. Journal of Speech, Language, and Hearing Research, 57(4), 1251-1258.
[https://doi.org/10.1044/2014_JSLHR-S-13-0188]

-
Martin, R. E., Goodyear, B. G., Gati, J. S., & Menon, R. S. (2001). Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiology, 85(2), 938-950.
[https://doi.org/10.1152/jn.2001.85.2.938]

-
May, N. H., Pisegna, J. M., Marchina, S., Langmore, S. E., Kumar, S., & Pearson, W. G., Jr. (2017). Pharyngeal swallowing mechanics secondary to hemispheric stroke. Journal of Stroke & Cerebrovascular Diseases, 26(5), 952-961.
[https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.11.001]

-
Miller, A. J. (2008). The neurobiology of swallowing and dysphagia. Developmental Disabilities Research Reviews, 14(2), 77-86.
[https://doi.org/10.1002/ddrr.12]

- Moore, K. L., & Dalley, A. F., II. (1999). Clinically oriented anatomy (4th ed.). Baltimore, MD: Lippincott Williams & Wilkins.
- Nogles, T. E., & Galuska, M. A. (2023). Middle cerebral artery stroke. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK556132/
-
Osawa, A., Maeshima, S., Matsuda, H., & Tanahashi, N. (2013). Functional lesions in dysphagia due to acute stroke: Discordance between abnormal findings of bedside swallowing assessment and aspiration on videofluorography. Neuroradiology, 55(4), 413-421.
[https://doi.org/10.1007/s00234-012-1117-6]

-
Park, T., Kim, Y., Ko, D.-H., & McCullough, G. (2010). Initiation and duration of laryngeal closure during the pharyngeal swallow in post-stroke patients. Dysphagia, 25(3), 177-182.
[https://doi.org/10.1007/s00455-009-9237-9]

-
Park. T., Kim, Y., & Oh, B.-M. (2017). Laryngeal closure during swallowing in stroke survivors with cortical or subcortical lesion. Journal of Stroke & Cerebrovascular Diseases, 26(8), 1766-1772.
[https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.04.003]

-
Perlman, A. L., Booth, B. M., & Grayhack, J. P. (1994). Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia, 9(2), 90-95.
[https://doi.org/10.1007/BF00714593]

-
Pisegna, J. M., & Langmore, S. E. (2018). The ice chip protocol: A description of the protocol and case reports. Perspectives of the ASHA Special Interest Groups, 3(13), 28-46.
[https://doi.org/10.1044/persp3.SIG13.28]

-
Robbins, J. A., & Levine, R. L. (1988). Swallowing after unilateral stroke of the cerebral cortex: Preliminary experience. Dysphagia, 3(1), 11-17.
[https://doi.org/10.1007/BF02406275]

-
Robbins, J. A., Levine, R. L., Maser, A., Rosenbek, J. C., & Kempster, G. B. (1993). Swallowing after unilateral stroke of the cerebral cortex. Archives of Physical Medicine and Rehabilitation, 74(12), 1295-1300.
[https://doi.org/10.1016/0003-9993(93)90082-L]

-
Rosenbek, J. C., Robbins, J. A., Roecker, E. B., Coyle, J. L., & Wood, J. L. (1996). A penetration-aspiration scale. Dysphagia, 11(2), 93-98.
[https://doi.org/10.1007/BF00417897]

-
Rosenbek, J. C., Roecker, E. B., Wood, J. L., & Robbins, J. A. (1996). Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia, 11(4), 225-233.
[https://doi.org/10.1007/BF00265206]

- Ruark, J. L., Mills, C. E., & Muenchen, R. A. (2003). Effects of bolus volume and consistency on multiple swallow behavior in children and adults. Journal of Medical Speech-Language Pathology, 11(4), 213-226.
- Snell, R. S. (2010). Clinical neuroanatomy (7th ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
-
Somasundaram, S., Henke, C., Neumann-Haefelin, T., Isenmann, S., Hattingen, E., Lorenz, M. W., & Singer, O. C. (2014). Dysphagia risk assessment in acute left-hemispheric middle cerebral artery stroke. Cerebrovascular Diseases, 37(3), 217-222.
[https://doi.org/10.1159/000358118]

-
Teismann, I. K., Dziewas, R., Steinstraeter, O., & Pantev, C. (2009). Time-dependent hemispheric shift of the cortical control of volitional swallowing. Human Brain Mapping, 30(1), 92-100.
[https://doi.org/10.1002/hbm.20488]

-
Teismann, I. K., Suntrup, S., Warnecke, T., Steinstrater, O., Fischer, M., Floel, A., . . . Dziewas, R. (2011). Cortical swallowing processing in early subacute stroke. BMC Neurology, 11, 34.
[https://doi.org/10.1186/1471-2377-11-34]

-
Wilmskoetter, J., Daniels, S. K., & Miller, A. J. (2020). Cortical and subcortical control of swallowing - Can we use information from lesion locations to improve diagnosis and treatment for patients with stroke? American Journal of Speech-Language Pathology, 29(2S), 1030-1043.
[https://doi.org/10.1044/2019_AJSLP-19-00068]

-
Wilmskoetter, J., Martin-Harris, B., Peason, W. G., Jr., Bonilha, L., Elm, J. J., Horn, J., & Bonilha, H. S. (2018). Differences in swallow physiology in patients with left and right hemispheric strokes. Physiology & Behavior, 194, 144-152.
[https://doi.org/10.1016/j.physbeh.2018.05.010]